Main content
Background – showing the gap
Childhood mortality causes around 3 million deaths annually in children under 5 years of age in low- and middle-income countries (LMICs). In sub-Saharan Africa (SSA) specifically, 1 in 13 children die before their fifth birthday – compared to 1 in 199 for high-income countries.[1] These deaths are largely preventable, as more than half of these deaths can be treated with simple, affordable interventions.[2,3,4]
To lower in-hospital morbidity and mortality, timely detection of deterioration and complications by (continuous) monitoring of vital signs is crucial. Children frequently exhibit only minimally visible symptoms, making them easy to be missed while they can rapidly progress into critical illness and death.[5] As an example, in the case of sepsis, an hour’s delay in treatment with antibiotics increases the mortality rate by 10%.[6] Thus, early detection and prediction of critical illness significantly improves the prognosis and chance of survival. Adequate monitoring is complicated in low resource settings (LRS) by shortage of staff and lack of suitable equipment.[7,8] A tool that can assist in early detection, prediction and proactive management of critical illness in a LRS could significantly improve the prognosis and chance of survival in LRS.
In July 2020, an international consortium of experts in critical care, social science, health economics, medical technologies, laboratory science, artificial intelligence and e-health joined forces with a social enterprise to develop a vital sign monitoring system, i.e. an affordable, durable, and user-friendly monitoring system (IMPALA) for hospitalised children in LRS.[9] The IMPALA consortium members are based in Malawi, the Netherlands and the UK, and we initially focussed on Malawi. In this article, we describe our consortium’s concept of monitoring, the challenges of monitoring in LRS that need to be overcome, our approach to the development so far, and some data leading up to our future plans.
Monitoring cascade
Monitoring in itself is not lifesaving, but is aimed at timely detection of a critical condition and should lead to early and effective action by health workers. We conceptualised this process into a model called the monitoring cascade (Figure 1). This model helps to understand what is needed for successful intervention and identifies potential gaps in this process. We use the model first to adjust the system design, addressing potential bottlenecks in the care process, and second to tailor the implementation to a specific hospital context.
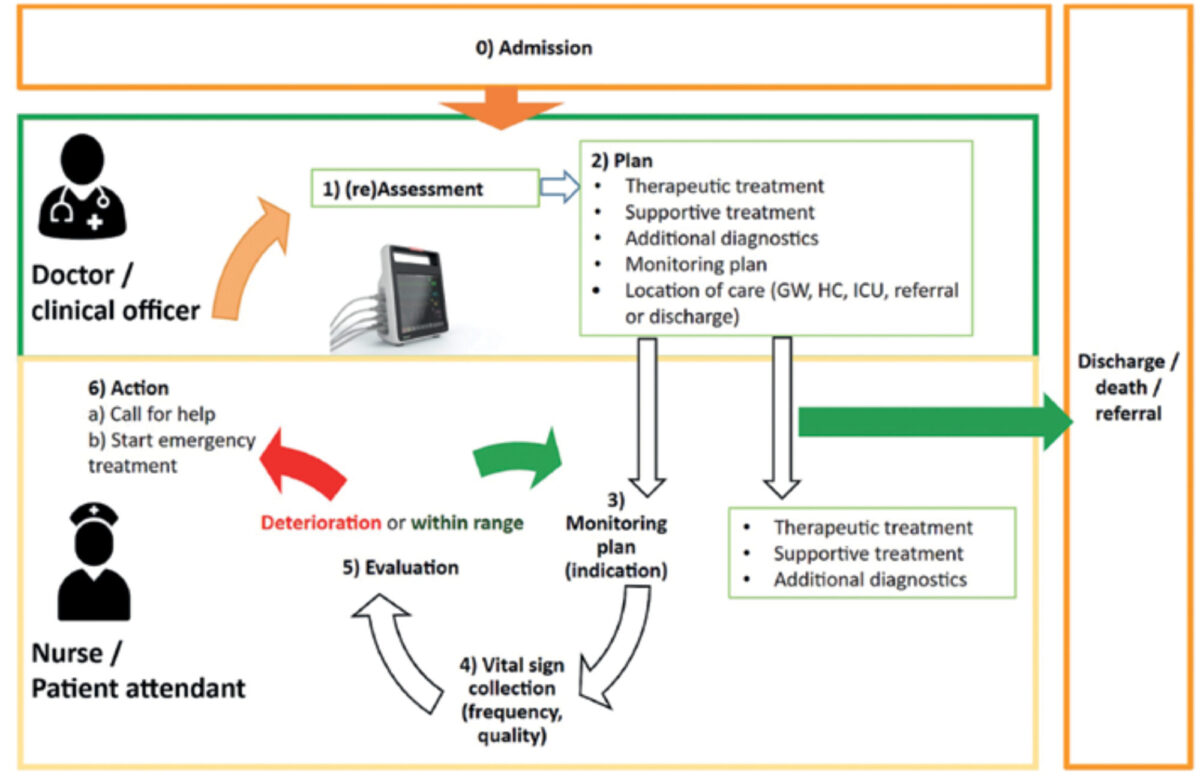
System requirements in lrs
Having worked in high-care and intensive care settings in low-income countries, we noticed that there is a lack of functioning vital signs monitoring systems, as most hospitals have no or just a few monitors. Even in situations where these systems are available or being introduced, several problems diminish the potential effect of these systems. We categorised the problems as follows:
- The systems used in high-resource settings (HRS) are too expensive for LRS. State-of-the-art systems produced by Philips, GE and WELCH can cost more than €16,000. Low-cost alternatives are less accurate, break down easily, and provide a poor user experience.
- In general, monitors cannot withstand harsh environments, break down easily, and cannot be repaired locally. As a result, up to 70% of medical devices do not work when they reach their destination in the LRS.[7,8]
- Current vital signs monitoring systems are not user-friendly and complex to use, requiring skilled staff to use them. This reduces adoption, increases the need for training, and limits the impact of these systems.
- Current systems detect at a late stage, which may be suitable for HRS with readily available staff and high-tech intensive care facilities, whereas LRS may benefit from earlier prediction and suggestion of therapies.
- Current monitoring systems do not consider the specific complexities of LRS – such as local culture, end-users, infrastructure, and staff.
Impala approach
1. Pilot study impala 1.0
This initial monitoring prototype consisted of a module from an existing monitor that records ECG, oxygen saturation, blood pressure and temperature. It additionally was equipped with a ballistography sensor, which was placed underneath the patient’s mattress and detects respiratory rate, heart rate and patient movement by sensing pressure changes. The sensor has the advantage that it is contactless, can be reused for months to years, and has the ability to also detect movement as a potential new vital sign. It was previously tested in a Dutch NICU and is widely used for monitoring adults during their sleep at home.[10,11] Supported by the NWO start-up grant, we conducted a pilot study in the paediatric Intensive care unit in Malawi, in which the monitor was compared with GE and Draeger monitoring systems to detect clinical accuracy and patient deterioration. In several cases, hours before it became clinically apparent, our prototype could detect clinical deterioration, such as sepsis, as changes in vital signs showed contemporary deteriorations. This pilot study further included usability tests and focus group discussion with nurses and doctors to identify the facilitators and barriers for monitoring in LRS. These data were used to develop an upgraded monitor for use in LRS (IMPALA 2.0, Figure 2b), selecting more suitable sensors, adjusting screen size, alarm limits, and battery life to match the conditions..
2. Consortium
The pilot also made us realise that for successfully designing and applying a monitor in a LRS we would require a broader expertise. We formed an international multidisciplinary consortium with experts in the field of critical care, social sciences, artificial intelligence, e-health and Point of Care laboratory diagnostics. This consortium consists of three Malawian partners (TRUE, KUHeS, MUBAS), three Dutch partners (AIGHD, NeLL, GOAL3) and one British partner (Imperial College London). with each bringing their own expertise. In this consortium, we tried to match experts in Europe and Malawi to work on projects to share knowledge in both directions. The EDCTP funding opportunity boosted the formation of the consortium as well as consecutive studies and monitor development.
3. Impala study
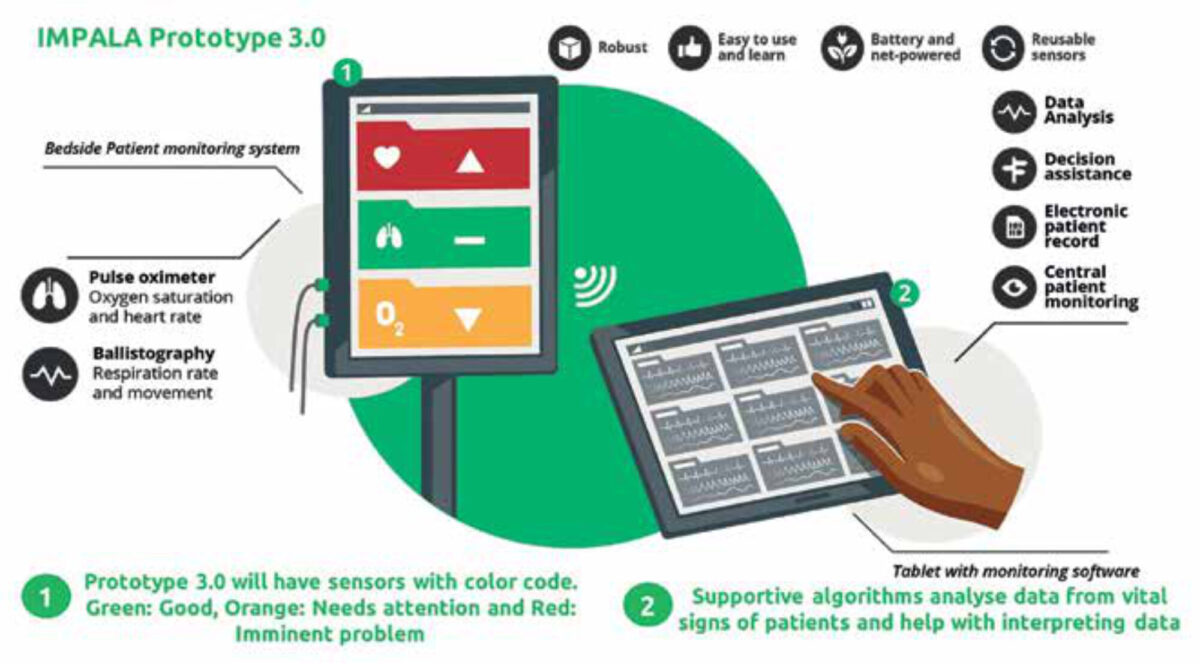
The main aim of the current stage of the IMPALA project is to further develop the IMPALA monitoring system to feature predictive algorithms based on vital signs, sociodemographic data, and biomarkers for early prediction of critical illness in children in LRS. This project was started in May 2021 and consists of three stages (Figure 3). In the first stage, the feedback from the pilot study using IMPALA 1.0 was used to develop an upgraded monitoring device (IMPALA 2.0), to perform a baseline clinical assessment prior to implementation and design and prepare the clinical studies. In the second stage IMPALA 2.0 was implemented and evaluated, and data was gathered for training and validation of the predictive algorithms for critical illness; this study is described in detail elsewhere.[12] The iterative design and usability allows for improvements of the monitor. In the third phase, IMPALA 3.0 will be developed through integration of the predictive algorithms and the final User Interface and hardware design. IMPALA 3.0 will then be evaluated in a mixed-methods pilot study to test the performance in terms of critical illness prediction and impact on clinical decision making.


4. Initial results
The clinical study was started in July 2022, as part of the IMPALA 2.0 stage, and at time of writing 500 patients have been recruited in the study. The study is aimed at registering Critical Illness Events, and so far a third of patients had one or more of these events recorded during their high dependency unit (HDU) admission. We chose to apply it in the HDU areas as PICUs are relatively scarce and, if present, already have monitors and a high nurse to patient ratio. The most commonly recorded events are respiratory events (31%) and mortality was 6.0%. The social science study noted several useful observations, such as the potential role of parents/guardians in the response to monitor alarms, the importance of implementation training for nurses and doctors, and several other facilitators and barriers of monitoring in LRS.
5. Future
The IMPALA 2.0 is based on a conventional monitoring system, and this is currently approved for use in Malawi. The social enterprise developing and manufacturing the monitor, GOAL3, has developed an implementation training using the lessons learned and has currently implemented the system successfully in the first hospital in Malawi. In Rwanda, the process of registration and licensing has started as well as an implementation study for assessing clinical impact.
The IMPALA project will lead to development of one or more algorithms, which will be implemented in the updated version IMPALA 3.0 (Figure 4). An implementation training and strategy is being created and will be included. Lastly, the search for biomarkers to detect and predict critical illness events may lead to additional predictors for disease severity and pathogen detection, which may be included in our algorithms and decision aids.
6. Conclusions
There is a need for early detection of potential need for treating critical illness events in hospitalised children in LRS. Appropriate monitoring in these settings cannot be achieved by just applying monitors used in HRS and requires an approach that is specifically designed to face the local challenges and needs . Using new techniques and a multidisciplinary consortium, we are trying to fill the gap and make a leap forward.

For more information
Go to: www.projectimpala.org, www.Goal3.org or mail to: Job.calis@gmail.com
Personal motivation of the author
Having set up an intensive care department in Malawi, I noticed that despite all available resources some children cannot be helped. Late detection of deterioration in the wards often led to irreversible presentations in PICU or even unnoticed deaths in the wards. A large proportion of this may be preventable if imminent deterioration is detected early and timely and adequate treatment is given. This may not only reduce mortality and morbidity but also reduce the resources needed, which are already limited. A lack of sufficient nursing staff in full wards, long night shifts, and lack of adequate critical care training to detect and treat these conditions were factors contributing to these late presentations. Monitors could potentially help the overstrained nurses, but I noticed that these were often lacking, required expensive sensors, often broke down beyond repair, were poorly responded to, and detected problems too late to allow early interventions. After returning to The Netherlands, Bart Bierling was introduced to me by my head of department of the PICU in the Amsterdam UMC (Job van Woensel). His ballistographic sensor and ideas to improve monitoring sounded like what was needed. Together with Niek Versteegde (AIGT), he started GOAL3, and the four of us were funded to start a pilot project in Malawi. The IMPALA consortium was a second step towards a full solution and our dream of leaving the frustration of being too late behind us and making an impact on child health care.
References
- Levels and Trends in Child Mortality – UNICEF DATA [Internet]. UNICEF DATA. 2019 [cited 6 August 2020]. Available from: https://data.unicef.org/resources/levels-and-trends-in-child-mortality/
- Children: reducing mortality [Internet]. Who.int. 2020 [cited 6 August 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality
- Child Mortality – UNICEF DATA [Internet]. UNICEF DATA. 2020 [cited 6 August 2020]. Available from: https://data.unicef.org/topic/child-survival/under-five-mortality/
- Ending preventable child deaths from pneumonia and diarrhoea by 2025 [Internet]. World Health Organization. 2013 [cited 6 August 2020]. Available from: https://www.who.int/maternal_child_adolescent/documents/global_action_plan_pneumonia_diarrhoea/en/
- Guideline: updates on paediatric emergency triage, assessment, and treatment: care of critically ill children [Internet]. Apps.who.int. 2016 [cited 6 August 2020]. Available from: https://apps.who.int/iris/bitstream/handle/10665/204463/9789241510219_eng.pdf
- Zaidi A, Ganatra H, Syed S, et al. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health. 2011;11(Suppl 3):S13. DOI: 10.1186/1471-2458-11-S3-S13.
- World Health Organisation. MEDICAL DEVICES: Managing the mismatch; WHO 2010, [cited 1 February 2020] http://whqlibdoc.who.int/publications/2010/9789241564045_eng.pdf
- Malkin, R. A. (2007). Barriers for medical devices for the developing world. Expert review of medical devices, 4(6), 759-763.
- IMPALA project; [Internet]. AIGHD; [Accessed on 26 March 2023]. Available from: https://www.projectimpala.org/
- Joshi R, Bierling BL, Long X, et al. A Ballistographic Approach for Continuous and Non-Obtrusive Monitoring of Movement in Neonates. IEEE J Transl Eng Health Med. 2018 Oct 12;6:2700809.
- Vesterinen V, Rinkinen N, Nummela A. A Contact-Free, Ballistocardiography-Based Monitoring System (Emfit QS) for Measuring Nocturnal Heart Rate and Heart Rate Variability: Validation Study. JMIR Biomed Eng 2020;5(1):e16620
- IMPALA study group; Innovative monitoring in paediatrics in low-resource settings: an aid to save lives? ISRCTN71392921 https://doi.org/10.1186/ISRCTN71392921 accessed on 26 mar 2023



















































