Main content
Most of us have expe-rienced the patient’s panic: “Doctor, some-thing is moving under my skin!” (Figure 1-3). The diagnosis of myiasis is easily made by those who are familiar with it, but many are not. Myiasis is a parasitic infes-tation of the skin with live larvae of a variety of fly species. It affects both humans and animals. It is more commonly seen in the tropics and is uncommon in temperate re-gions.[1,2] The parasite is usually seen in tourists after returning home; in low- and middle-income countries (LMICs) it may affect people of all socio-economic classes; many are familiar with the condition and do not report to a health facility unless there are complications. The true prevalence is not known. Myiasis (derived from the Greek myia = fly) refers to the invasion of human or animal tissues by the maggots of flies. Different body parts may be involved, resulting in different clini-cal classifications, e.g., wound, cu-taneous, dermal, traumatic, gastric, rectal, and genitourinary myiasis. The larvae feed on tissue (live or dead), or in the case of intestinal myiasis, on ingested food.
Human myiasis can present as:[1,2]
- Obligate or mandatory – here, myiasis refers to true parasitism, where part of the developmental stage of the larvae is spent in living tissue. Most commonly, this occurs in animals such as sheep, cattle, and horses, but the invasion of human tissue by the human botfly in Central and South America and the mango fly in Africa has been well documented.
- Optional or facultative – myia-sis occurs when larvae invade living tissue after lodging in nearby, organic, or decay-ing tissue in a wound.
- Accidental – myiasis (pseudo-myiasis) refers to larvae found in the gut or urinary tract. These larvae are usually accidentally ingested with food. There is no obligate intestinal dipterous parasite of humans. Although usually a benign event, abdominal pain, nausea, and vomiting can result from accidental myiasis.[1,2]
Transmission
Many different fly larvae (maggots) are involved in myiasis; identifica-tion can be complex and requires an entomologist. The causative agent of myiasis varies between geographical regions: the bot fly Dermatobium hominis is most frequent in the Americas, while in Africa the blow fly Cordylobia anthropophaga is the most common. Symptoms caused by the maggots of these different fly species are similar.
D. hominis
The size of the D. hominis fly is 12-18 mm; it is densely haired and looks like a bumblebee.[3] It occurs in the neo-tropical regions of the New World, from southern Mexico to northern Argentina, where temperature and humidity are relatively high, in mainly lowland for-ests, especially in trails along forest and scrub. It is particularly important as a cause of myiasis in cattle in Brazil.[1,3,4]
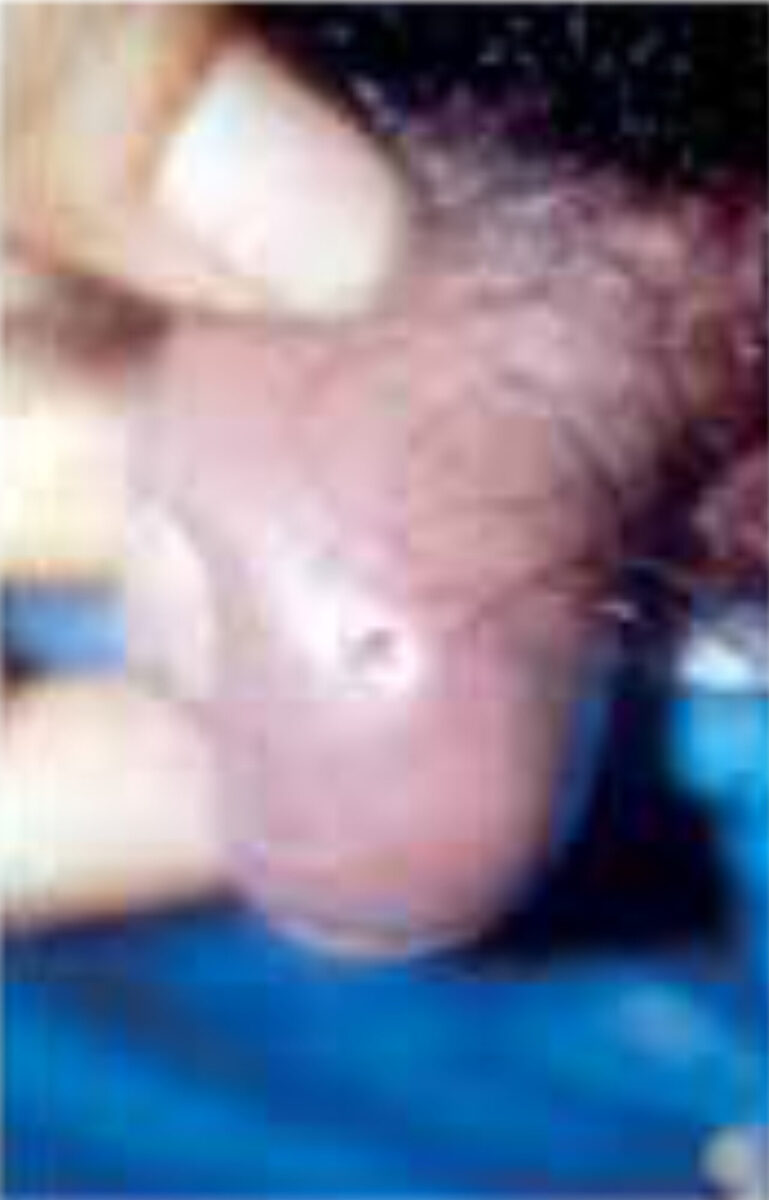
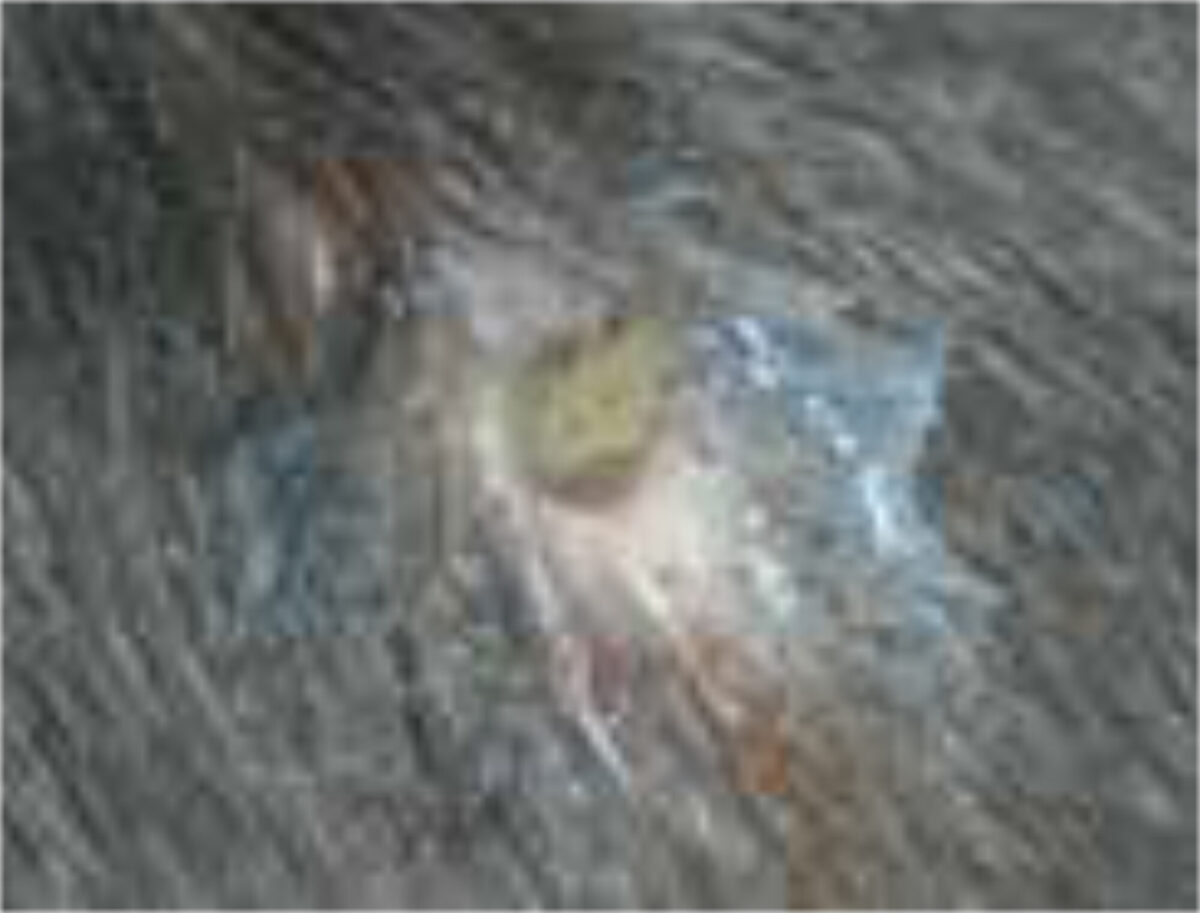
The female fly sticks approximately 6-30 eggs on to the body of other insects such as day-flying mosquitoes, blood-sucking flies and even ticks, which then serve as vectors to carry the eggs to the host (a process known as phoresy). The female fly deftly grabs the insect vector in mid-air and deposits eggs on its abdomen. Embryos begin to develop into first instar (the first stage in development) larvae but refrain from hatching until the vector lands and feeds on a potential host. The larvae then emerge and within ten minutes burrow into the dermis and take two days to reach the subcutaneous tissues. This results in furuncle-like skin lesions with an opening, through which the larvae breathe (Figure 2). The development lasts approximately 40-60 days, after which the larvae emerge, drop to the ground, and pupates.[1,3]
C. anthropophaga
This blow fly occurs in Africa and is often called the putzi, tumbu or mango fly. It belongs to the family of blow flies that frequently cause furuncles that may coalesce, and plaque forma-tion. Adults are relatively large (9-12 mm); they are stout, compact flies with a yellow to light brown colour and two dark gray, ill-defined dorsal longitudinal thoracic stripes.[1,5]
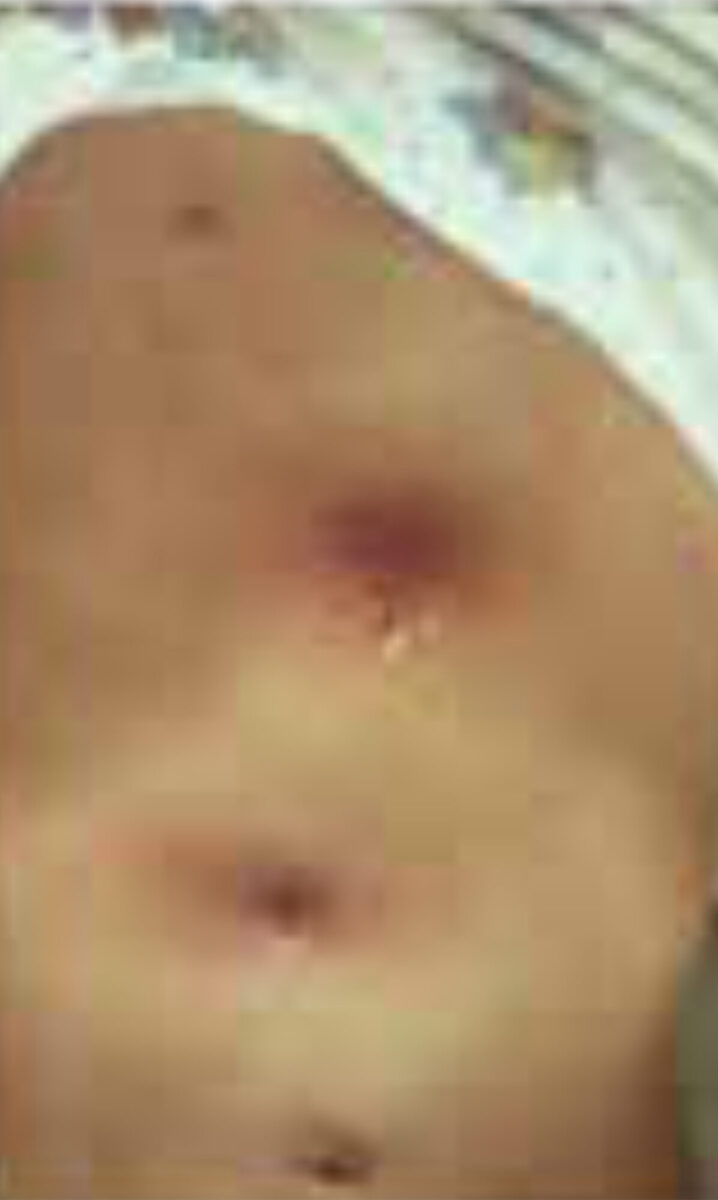
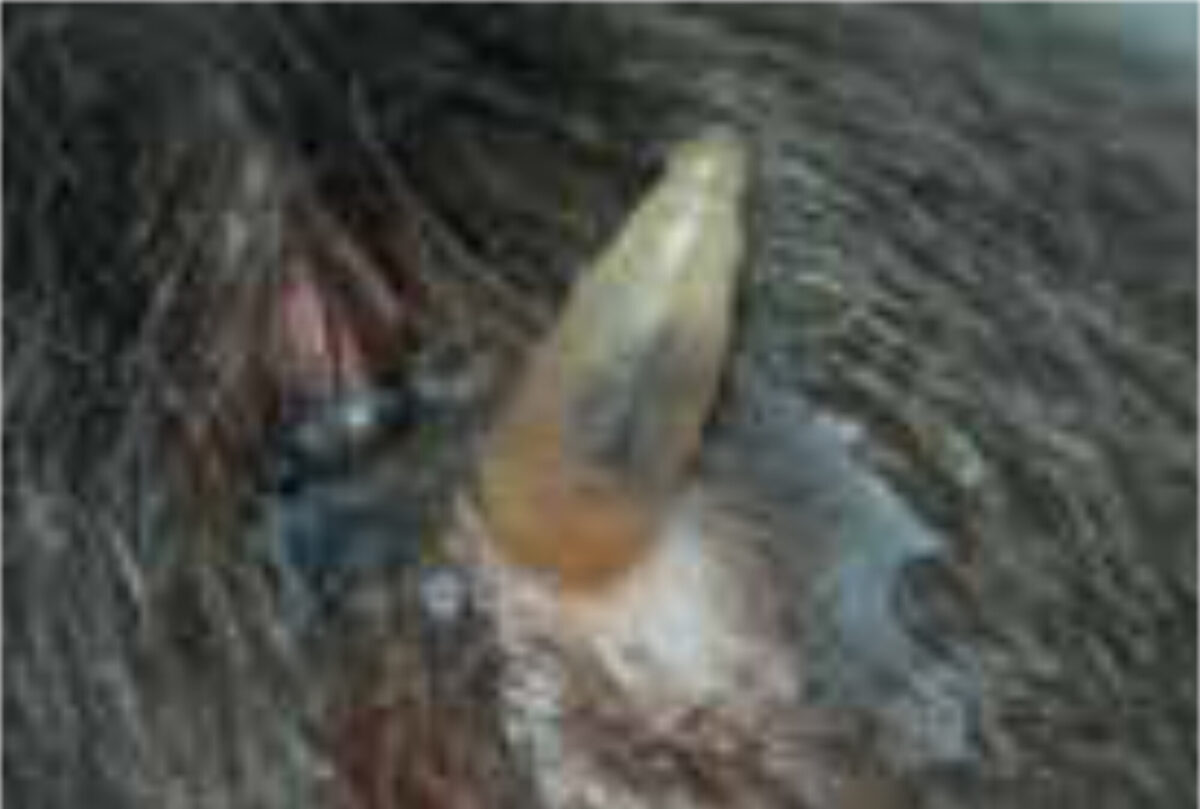
C. rodhaini (Lund’s fly) is the only other Cordylobia species known to infest humans. It has a limited distribution in Africa, mainly the tropical rainforests. It often causes more than one lesion, lead-ing to extensive furuncular myiasis.[1,6] Females of all Cordylobia species can lay up to 100-300 white banana-shaped eggs on sand or soil in shady areas, especially if contaminated with urine or faeces such as baby’s diapers as well as laundry drying outside in the sun. The larva uses its powerful oral hooks to quickly penetrate the skin, leaving only the rear part of its abdomen in contact with the air (Figure 3). When development is complete (usually within 7-14 days) it leaves the host and falls to the ground, where it buries itself and pupates.[1,5]
Clinical features, diagnosis and treatment
Obligate or mandatory myiasis
This is a zoonosis caused by D. hominis and Cordylobia species; humans are not accidental hosts. The most common clinical form is the furuncular form, in which a boil-like lesion develops gradually over a few days. After infec-tion, there may be mild constitutional symptoms. Each lesion has a central punctum, which drains serosangui-nous fluid. The larva quickly burrow itself into the skin, but leave its pos-terior end, which contains a group of spiracles, in direct contact with the air. The movements are usually noticed by the patient, as is the associated pain. Lymphangitis and regional lymphade-nopathy may result from the associated inflammatory response; eosinophilia may be found in the blood. Once the larva has emerged or is removed, the lesions quickly disappear.[1,2]
Diagnosis
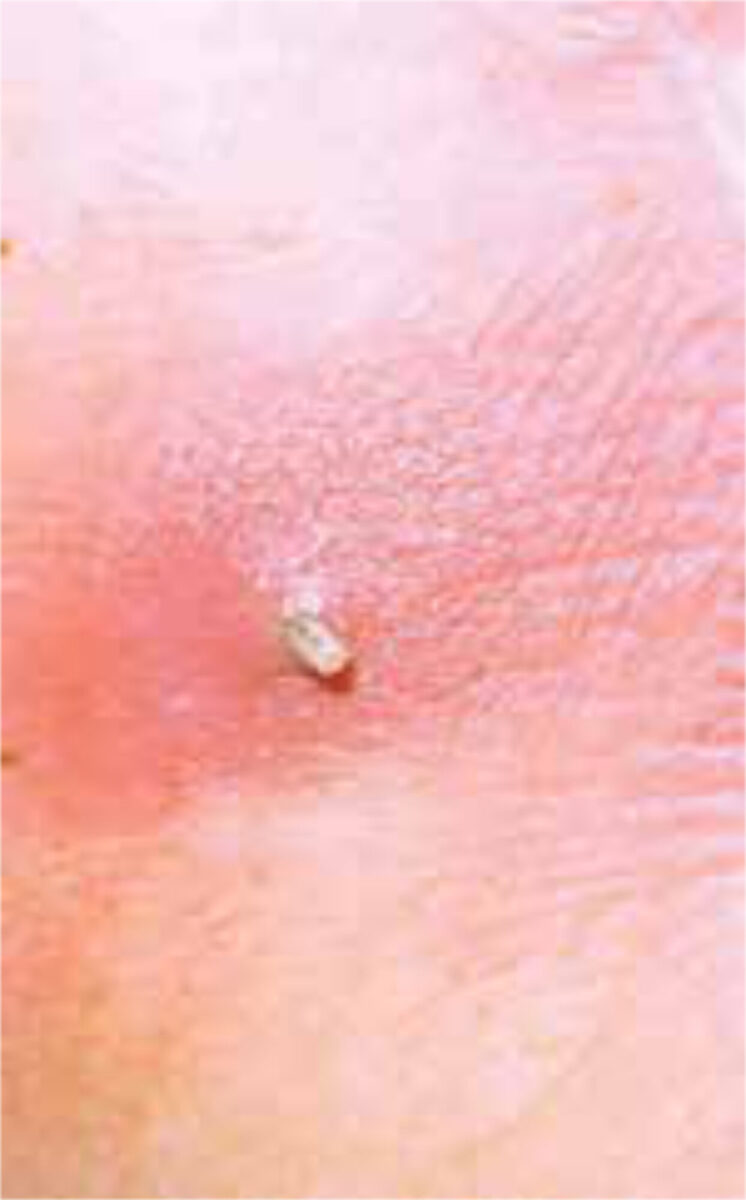
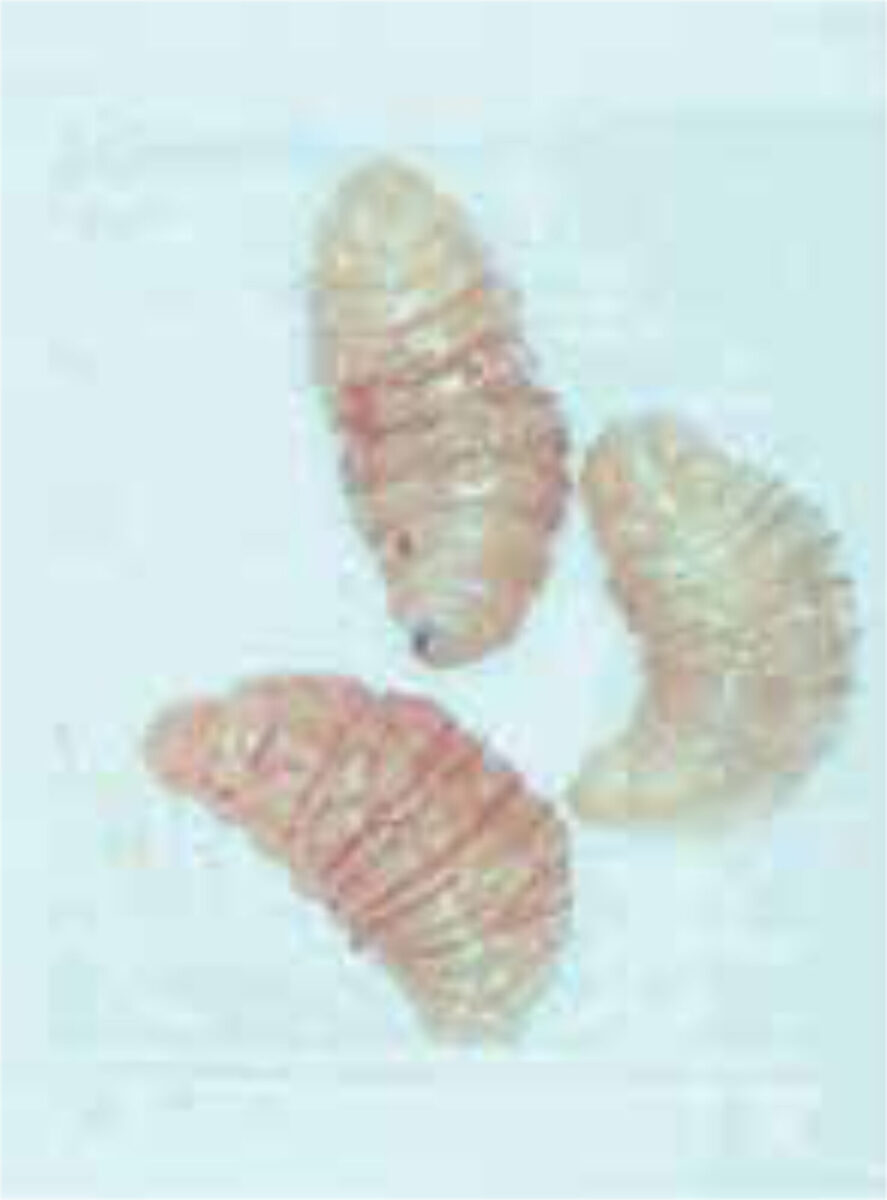
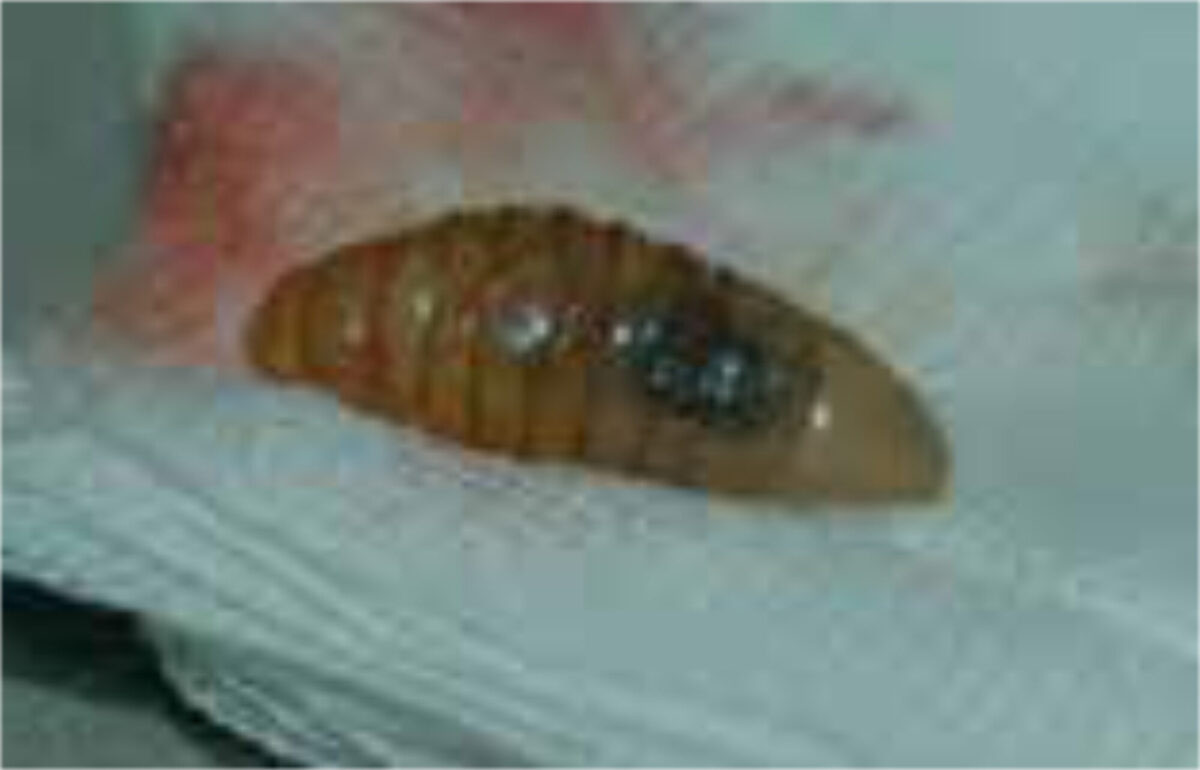
The diagnosis is usually supported by the travel history, the presence of boil-like lesions in which the patient feels movement, and lack of response to antibiotic treatment (Figure 3).[7,8] Ultrasonography may be helpful.[9] The differential diagnosis includes staphylococcal skin infections, cat-scratch disease, insect bites, tick-bite granuloma, inflamed cysts and tungi-asis.[1] It is important to identify the larvae and determine whether they are facultative or obligate parasites. They are best killed by immersing it in very hot (>80C°), and then pre-served in a solution of 70-95% ethanol. Formalin solution should not be used for preservation as it causes hardening of the larval tissue, which adversely affects processing (Figure 4 A,B).[12]

Treatment
The maggots can sometimes be expressed by firm pressure around the edges of the lesions, but the punctum may require surgical enlargement. This doesn’t work so well for the D. hominis larva, which has a bulbous tip and is equipped with rows of backward-facing spines. Traditional methods of treat-ment include sealing the punctum with pork fat, which blocks the larva’s breath-ing hole and encourages extrusion.[13] The same principle can be achieved with mineral oil, petroleum jelly (Vaseline™), butter, or a transparent occlusive dress-ing (Figure 5 A-D).[14] Occlusion may be less effective in the advanced stages of the infestation; surgical treatment is then the preferred choice. The lesions are hardly ever infected, therefore in general antibiotics are not needed.
Optional or facultative myiasis
Fannia canicularis (small house fly) and Musca domestica (house fly) can lay their eggs in wounds, ulcers, and moist areas, giving rise to facultative myiasis in wounds and urogenital myiasis. Urogenital myiasis occurs when ovipositing flies lay their eggs near genital orifices, through which larvae enter the genital tract, causing pain, and larvae may be found by accident in the urine (accidental myiasis).[1] Clinical features depend on the locali-sation. Facultative myiasis is not an uncommon complication of wounds in LMICs. While these are commonly traumatic, these also occur in patients with leprosy or elephantiasis (caused by lymphatic filariasis). Wounds that are exposed (not covered) in hot weather are most at risk. The larvae (mag-gots) can be seen, sometimes in large numbers, in the purulent tissues, and their removal of necrotic tissue and beneficial effect on granulation has led to their use in maggot debridement therapy.[15,16] The difficulty is in contain-ing them in the wound as some maggots prefer healthy granulation tissue.
Therapy
Debridement and irrigation should be done with treatment of second-ary infection.[17] Ivermectin has been used in wound infestation (topical and oral) and in the management (oral) of cavitary myiasis, such as aural or nasal infestation, where manual removal of larvae is painful.[18]
Prevention
Prevention is by sleeping in-doors, window screens, repellents, protec-tive clothing, and sanitation. Laundry should be ironed as this kills the larvae. Wounds need to be cleaned and covered.
Accidental – myiasis (pseudo-myiasis)
This form of myiasis can be related to accidental ingestion of eggs or larvae on contaminated food or water. Symptoms of accidental myiasis are nonspecific. Usually there is abdominal pain, nausea, and rectal bleeding, as well as toxigenic or bacterial gastro-enteritis. The diagnosis is by clinical suspi-cion. Larvae can be observed in stool specimen examination. Treatment with oral ivermectin could be tried but nonspecific supportive measures are more helpful.[1] Prevention is also here most important. Exposed food should be discarded, and fruits and vegetables should be washed prior to consumption. Meat should be fresh and free of eggs and larvae.
References
- Kapoor R, McGraw TA, Elston DM. Myiasis. In: Faber WR, Hay RJ, Naafs B, editors. Imported skin. 2nd ed. Chichester: Wiley-Blackwell; 2013. p. 252-63
- Goddard J. Infectious diseases and arthropods. 3rd ed. Cham: Humana Press; 2018. p. 294
- Kahn DG. Myiasis secondary to Sermatobia hominis (human Botfly) presenting as a long-standing breast mass. Arch Pathol Lab Med. 1999 Sep;123(9):829-31. doi: 10.5858/1999-123-0829-MSTDHH
- Das Neves JH, Carvalho N, Amarante AFT. Dermatobia hominis: potencial risk of resistance to macrocyclic lactones. Vet Parasitol. 2015 Sep 15;212(3-4):483-6. doi: 10.1016/j.vetpar.2015.06.029. Epub 2015 Jul 2
- Scholl PJ, Colwell DD, Cepeda-Palacios R. Myiasis (Muscoidea, Oestroidea). In: Mullen GR, Durden LA, editors. Medical and veterinary entomology. 3rd ed. Cambridge, Academic Press, 2019. p. 383-419
- Zumpt F. Myiasis in man and animals in the Old World: a textbook for physicians, veterinarians, and zoologists. London: Butterworths; 1965. p. 297
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995 May:132(5):811-4. doi: 10.1111/j.1365-2133.1995.tb00732.x
- Maier H, Hönigsmann H. Furuncular myiasis caused by Dermatobia hominis, the human botfly. J Am Acad Dermatol. 2004 Feb;50(2 Suppl):S26-S30. doi: 10.1016/S0190-9622(03)01488-9
- Bowry R, Cottingham RL, Use of ultrasound to aid management of late presentation of Dermatobia hominis larva infestation. J Accid Emerg Med. 1997 May;14(3):177-8. doi: 10.1136/emj.14.3.177
- Amedt J, Campobasso CP, Gaudry E, et al. Best practice in forensic entomology-standards and guidelines. Int J Legal Med. 2007 Mar;121(2):90-104. doi: 10.1007/s00414-006-0086-x
- Adams ZJO, Hall MJR, Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci Int. 2003 Dec 17;138(1-3):50-61. doi: 10.1016/j.forsciint.2003.08.010
- Schauff ME, editor. Collecting and preserving insects mites: techniques and tools. Washington: Systematic Entomology Laboratory, USDA, National Museum of Natural History; 2001. p. 68
- Brewer TF, Wilson ME, Gonzalez E, et al. Bacon therapy and furuncular myiasis. JAMA. 1993 Nov 3;270 (17):2087-8
- Kennedy C, Kazzaz GAA, Van Diggelen MW, Naafs B. Myiasis, een therapeutisch pareltje. NTVDT. 2001;11:16
- Guicheney P. Réhabilitation de l’asticot. Nouv Presse Med. 1980 Mar 15;9(13):964
- Moya-López J, Costela-Ruiz V, García-Recio E, et al. Advantages of maggot debridement therapy for chronic wounds: a bibliographic review. Adv Skin Wound Care. 2020 Oct;33(10):515-25. doi: 10.1097/01.ASW.0000695776.26946.68
- Jelinek T, Northdurft HD, Rieder N, et al. Cutaneous myiasis: review of 13 cases in travelers returning from tropical countries. Int J Dermatol. 1995 Sep;34(9):624-6. doi: 10.1111/j.1365-4362.1995.tb01088.x
- Singh S, Athar M, Chaudhary A, et al. Effect of ivermectin on wound myiasis: a hospital based study. Ann Clin Lab Res. 2017;5(4);200. doi: 10.21767/2386-5180.1000200


















































