Main content
Tungiasis is a common condition in the tropics, also known as sand flea disease or jiggers. It is caused by the penetration of the female sand flea Tunga penetrans into the epidermis of its host. It is a huge problem in neglected areas where affected persons, particularly children, can develop pain and deformities of the feet that lead to difficulty in walking. Tungiasis is often treated at the level of local healers and most patients are familiar with the condition. It is only seen by doctors or by dermatologists when it occurs in the higher socio-economic class, or in tourists who do not know the condition and have terrible itching. Although patients and local healers know how to treat the condition, complications such as bacterial su-perinfection can occur that require medical care.
Epidemiology and risk factors
Tungiasis is found on the American continent, from Mexico to northern Argentina, on several Caribbean islands, and in almost every country in Sub-Saharan Africa. In endemic countries, the distribution of tungiasis is uneven and usually occurs in foci. Typically, these are urban squatter settlements, traditional villages along the coast, or underdeveloped communities in the rural hinterland, often in places rarely visited by the mainstream tourist.[1,2] Tungiasis is a zoonosis that affects a wide variety of domestic and wild animals. Depending on the setting, dogs, cats, pigs, and rats are the main reservoirs for the sand fleas.[3,4]
The condition is considered a neglected disease of marginalised populations. In resource-poor com-munities, the prevalence can be as high as 60%.[5] Individuals can har-bour between a few to more than a hundred sand fleas.[6] There is a clear seasonality in the incidence of new penetrations, with little occurrence during the rainy season and a high attack rate during the dry season.[1,8]

While sandy soil and the beach is where most tourists become infected,[9] sand fleas also reproduce easily on any other type of soil, in banana plantations, and in compounds. Even dust-filled crev-ices in a floor are suitable sites for host propagation, provided there is some organic matter for larvae to feed on and the soil temperature is high enough to allow development of the egg into an adult flea.[1,4] The infestation with the sand fleas occurs when walking barefoot or when bare skin touches the ground where adult sand fleas are present: on a beach, on unpaved paths, around the houses in a compound, or in houses without a solid floor.[4] After a sand flea has found a suitable site, usually the toes, under the nails, between the sole and the toes, the heel, or the lateral edge of the foot, it invades the stratum corneum and is even fully entrenched in the epidermis in less than thirty minutes to a few hours.[2] The penetra-tion process is usually not noticed.[1]
Pathophysiology
Once the sand flea is embedded in the stratum corneum of the skin, the flea undergoes development, causing the abdominal segments to enlarge to the size of a pea, loaded with eggs. Hundreds of eggs are released through a minimal opening in the skin in a period of about three weeks.[1,2] After the eggs are released, the involution of the expanded abdomen begins. Three to four weeks after penetra-tion, the parasite dies and is eventually removed from the epidermis by the tissue repair mechanisms. Tungiasis is a self-limiting condition.[1,2]
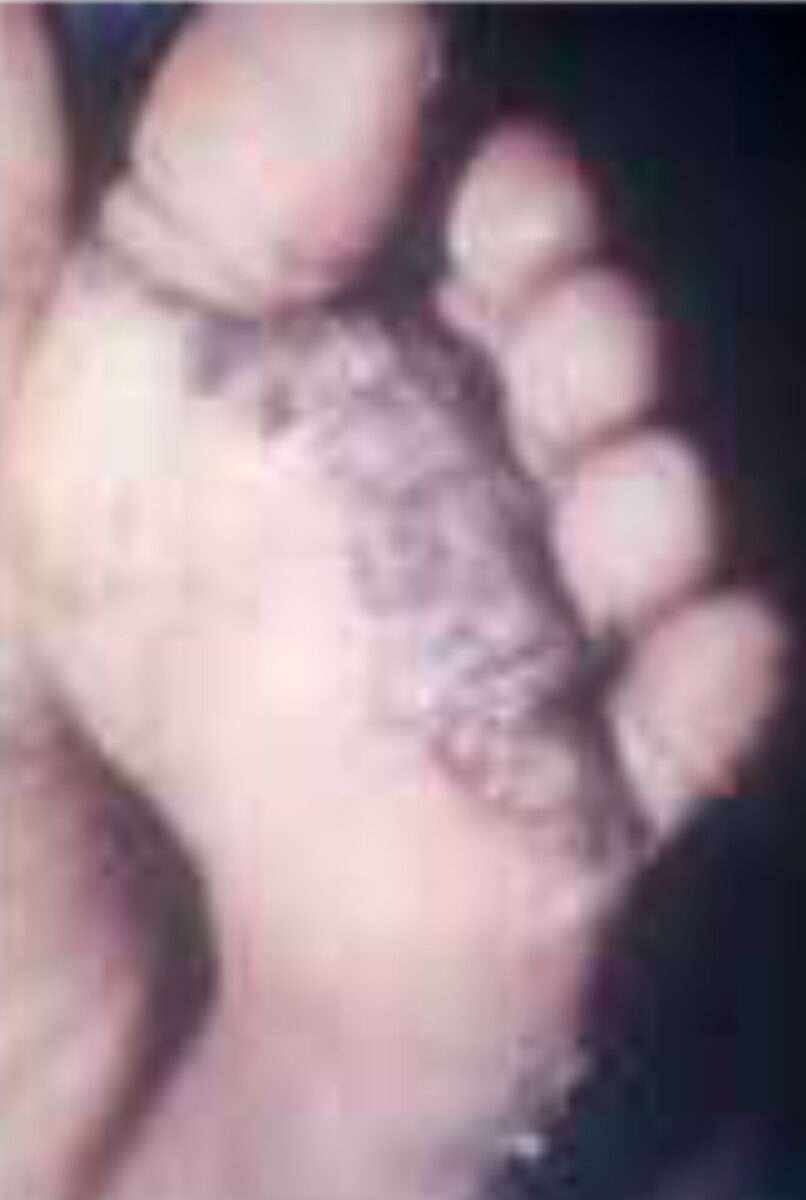
However, in poor individuals living in endemic areas reinfection is the rule, and its consequences are common. Repeated infection leads to chronic inflammation of the feet with persis-tent pain and difficulty in walking (Figure 1).[6] In these settings, tungiasis can lead to significant morbidity and impairment in quality of life.[7] Because it is relatively rare among tourists and the local middle-to-upper class, the condition is often misdiagnosed, and patients are subjected to inap-propriate therapeutic procedures.[1,2]
Clinical features
It is important to realize that tungiasis is a dynamic process.[10] Consequently, the macroscopic appearance of tungiasis depends on the stage of the maturing flea. Based on clinical and morphologi-cal criteria, the natural course of tungi-asis can be divided into five stages:[1,2,10]
- Stage I (flea in statu penetrandi, thirty minutes to several hours). A small reddish spot of about one millimetre appears with or without an erythematous halo.
- Stage II (onset of hypertrophy, 1-2 days after penetration). The lesion becomes more apparent as a growing papule develops. The protruding back of the flea with the anal-genital opening appears as a central black dot surrounded by erythema. At this stage, there may be severe itch-ing, especially in patients exposed to the parasite for the first time.
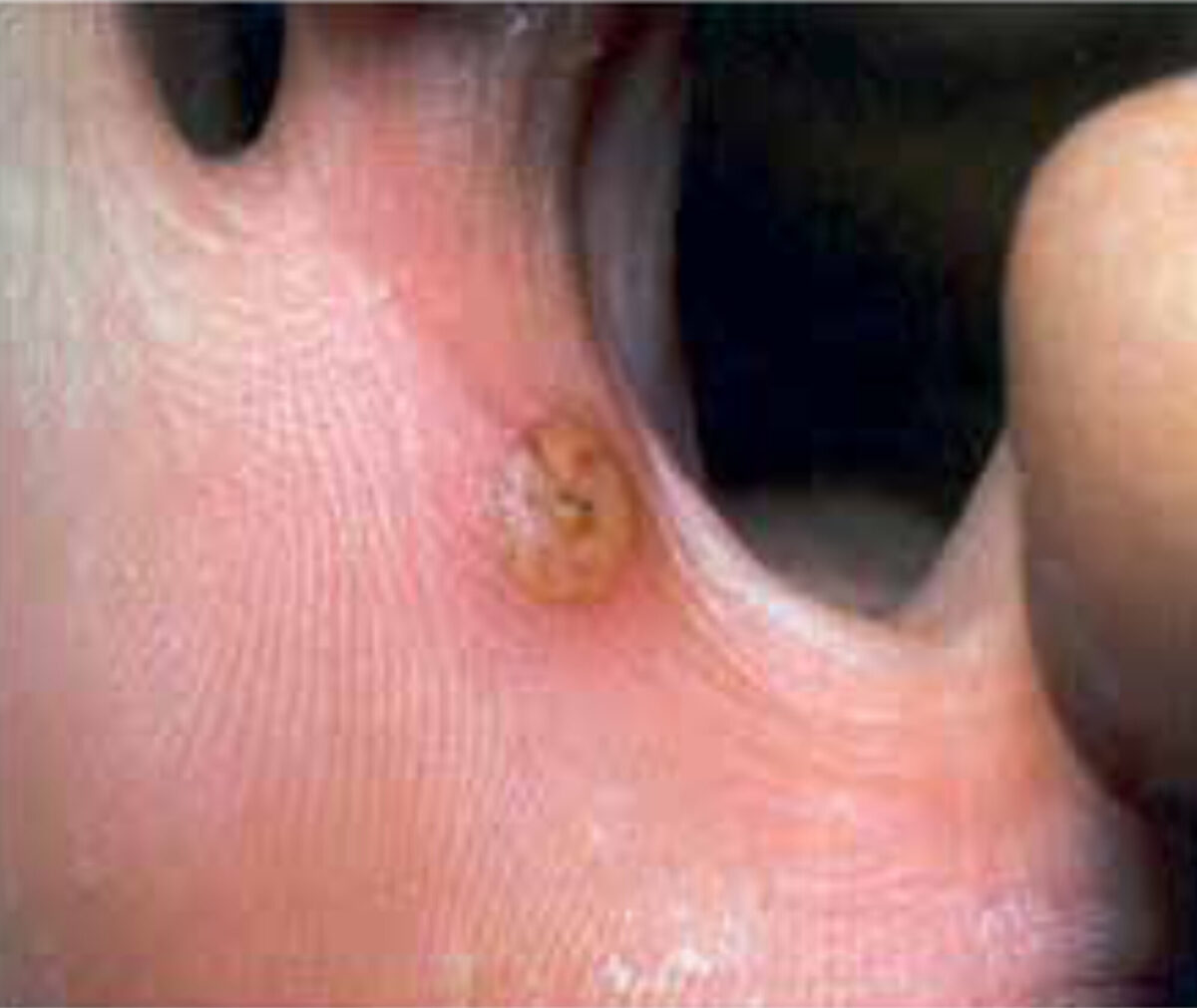
- Stage III (maximal hypertrophy, two days to three weeks after penetration). The hypertrophy becomes visible macroscopically. A round elevation with well-defined edges and tight consistency appears, frequently surrounded by desquamation. Eggs and faeces production is typical at this stage, and the lesion is painful (Figure 2).
- Stage IV (3-5 weeks after pen-etration). A black crust covers an involuted lesion with a dead, decaying parasite. At the end of this stage the carcass of the ectoparasite disappears from the epidermis resulting in a circu-lar impression in the skin.
- Stage V (six weeks to several months after penetration). Characteristic is the stamp-shaped imprint in the stra-tum corneum (Figure 1).
In persons who are repeatedly infected, the lesions will be in one of these five stages. This natural history of sand flea disease may be complicated by bacterial superinfection and manipulation of the lesions by the patient or his caregiver. Bacteria are either introduced into the epidermis by the invading flea or introduced by scratching or attempting to remove the flea with a non-sterile instrument. In endemic areas, bacterial superinfection is present in almost all cases, while it is less common among tourists.[4,11] Superinfection leads first to a micro abscess, then to a pustule, and finally to suppuration. Staphylococcus aureus and streptococci are the most common micro-organisms isolated, but other aerobic and anaerobic bac-teria (including Clostridiae) are also found.[11] Importantly, this can lead to tetanus in unvaccinated individuals.
Pathogenic micro-organisms can enter the circulation as the parasite’s pro-boscis is inserted into a capillary of the dermis. If the flea is removed with a sharp instrument such as a needle, nail, or thorn, it leaves a small wound that easily becomes superinfected. When the parasite ruptures during manipulation or if the mouth part remains in the dermis, a foreign body inflammatory reaction may develop.[1]
Diagnosis
The diagnosis of tungiasis is made clini-cally. For tourists and individuals not liv-ing in endemic areas, the travel history is of importance. The patient complains about severe local itching, pain, and the sensation of something in the skin. The presence of two or more identical lesions at the toes, particularly along the nail rim, is diagnostic. The observa-tion of eggs being expelled or attached to the skin around the lesion and the release of brownish threads of faeces are pathognomonic signs. The use of a dermatoscope is helpful.[12] Expulsion of eggs can be provoked by massaging the hypertrophy zone slightly. While the feet are predilection sites for tungiasis, one should be aware that other parts of the body can be involved too.[1] The differ-ential diagnosis differs according to the stage. Skin biopsy is hardly ever needed.
Treatment
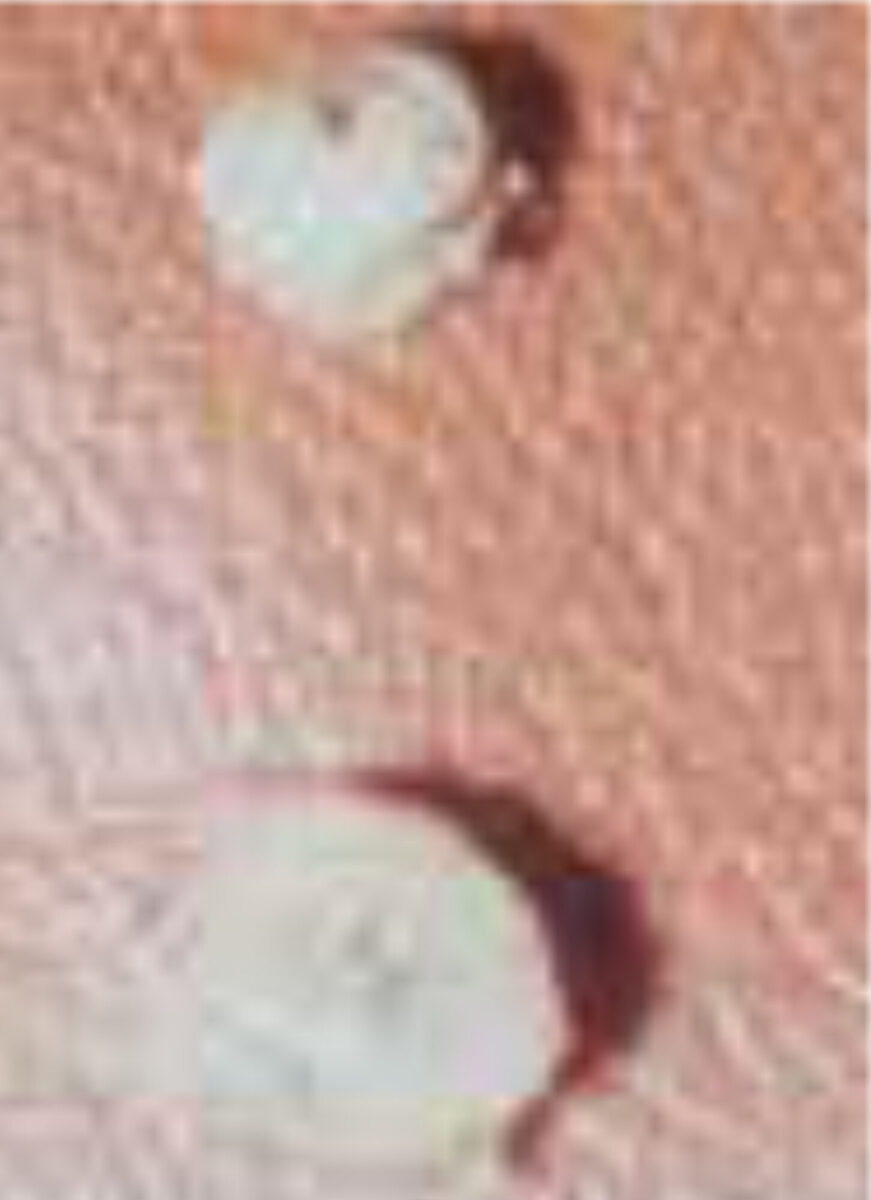
Surgical extraction of the flea under sterile conditions is still the only reli-able treatment. The opening in the epidermis must be widened until the abdomen is completely freed (Figure 3). Then the entire flea must be carefully taken out with tweezers (Figure 4). After the extraction of the parasite, the wound should be treated with an antiseptic or, according to some, with a topical antibiotic. The tetanus immune status must be checked. A randomised controlled trial has shown that oral ivermectin is not effective in the treatment of tungiasis.[13] However, there is sufficient evidence supporting the use of occlusive agents, especially dimeticone-based products.[14,15]
Prevention
Wearing socks and closed shoes protects to a certain degree. Daily inspection of the feet and immediate extraction of embedded fleas prevents complications. A twice-daily application of a repellent based on plant extract (jojoba seeds) in coconut oil (Zanzarin®), reduced the infestation rate in an area with an intense transmission by almost 90%.[16,17] If applied regularly, it protects effectively against invading sand fleas, even if no shoes are worn. Unfortunately, for children in low-income coun-tries this is at present still a dream. Commercial Zanzarin® is not produced any more, but it can be made locally.
References
- Feldmeier H, Heukelbach J. Tungiasis. In: Faber WR, Hay RJ, Naafs B, editors. Imported skin diseases. 2nd ed. Chichester: Wiley-Blackwell; 2013. P. 234-42
- Eisele M, Heukelbach J, Van Marck E, et al. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol Res. 2003 Jun;90(2):87-99. doi: 10.1007/s00436-002-0817-y
- Heukelbach J, Costa AML, Wilcke T, et al. The animal reservoir of Tunga penetrans in severely affected communities in north-east Brazil. Med Vet Entomol. 2004 Dec;18(4):329-35. doi: 10.1111/j.0269-283X.2004.00532.x
- Linardi PM, Calheiros CML, Campelo-Junior EB, et al. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann Trop Med Parasitol. 2010 Jun;104(4):337-45. doi: 10.1179/136485910X1274355475990d
- Feldmeier H, Sentongo E, Krantz I. Tungiasis (sand flea disease): a parasitic disease with particular challenges for public health. Eur J Clin Microbiol Infect Dis. 2013 Jan;32(1):19-26. doi: 10.1007/s10096-012-1725-4
- Feldmeier H, Eisele M, Sabóia-Moura RC, et al. Severe tungiasis in underprivileged communities: case series from Brazil. Emerg Infect Dis. 2003 Aug;9(8):949-55. doi: 10.3201/eid0908.030041
- Wiese S, Elson L, Feldmeier H. Tungiasis-related life quality impairment in children living in rural Kenya. PLoS Negl Trop Dis. 2018 Jan 8;12(1):20005939. doi: 10.1371/journal.pntd.0005939
- Heukelbach J, Wilcke T, Harms G, et al. Seasonal variation of tungiasis in an endemic community. Am J Trop Med Hyg. 2005 Feb;72(20:145-9
- Heukelbach J, Gomide M, Araújo Jr F, et al. Cutaneous larva migrans and tungiasis in international travelers exiting Brazil: an airport survey. J Travel Med. Nov-Dec 2007;14(6):374-80. doi: 10.1111/j.1708-8305.2007.00156.x
- Feldmeier H, Eisele M, Van Marck E, et al. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: IV. Clinical and histopathology. Parasitol Res. 2004 Oct;94(4):275-82. doi: 10.1007/s00436-004-1197-2
- Feldmeier H, Heukelbach J, Eisele M, et al. Bacterial superinfection in human tungiasis. Trop Med Int Health. 2002 Jul;7(7):559-64. doi: 10.1046/j.1365-3156.2002.00904.x
- Flajoliet N, Bertolotti A. Potential use of dermoscopy in atypical tungiasis. J Travel Med. 2021 Aug 27;28(6):taab072. doi: 10.1093/jtm/taab072. Epub ahead of print
- Heukelbach J, Franck S, Feldmeier H. Therapy of tungiasis: a double-blinded randomized controlled trial with oral ivermectin. Mem Inst Oswaldo Cruz. 2004 Dec;99(8):873-6. doi: 10.1590/50074-02762004000800015
- Abrha S, Heukelbach J, Peterson GM, et al. Clinical interventions for tungiasis (sand flea disease): a systematic review. Lancet Infect Dis. 2021 Jul 5:S1473-3099 (20)30853-7. doi: 10.1016/S1473-3099(20)30853-7. Epub ahead of print
- Tardin Martins AC, De Brito AR, Kurizky PS, et al. The efficacy of topical, oral and surgical interventions for the treatment of tungiasis: a systematic review of the literature. PLoS Negl Trop Dis. 2021 Aug 20;15(8):20009722. doi: 10.1371/journal.pntd.0009722
- Feldmeier H, Kehr JD, Heukelbach J. A plant-based repellent protects against Tunga penetrans infestation and sand flea disease. Acta Trop. 2006 Oct;99(2-3):126-36. doi: 10.1016/j.actatropica.2006.05.013
- Buckendahl J, Heukelbach J, Ariza L, et al. Control of tungiasis through intermittent application of a plant-based repellent: an intervention study in a resource-poor community in Brazil. PLoS Negl Trop Dis. 2010 Νον 9;4(11):e879. doi: 10.1371/journal.pntd.0000879


















































