Main content
Setting
The rural San Carlos Hospital is located in Altamirano, a small town in the south of Mexico bordering the jungle of Chiapas. This nonprofit mission hospital provides health care primarily to the poor indigenous population of the region. The staff consists of Mexican ‘primary care doctors’, a surgeon and an internist. It has basic laboratory facilities, an X-ray machine and an ultrasound apparatus. The nearest regional referral hospital is three hours away by car.
Case report
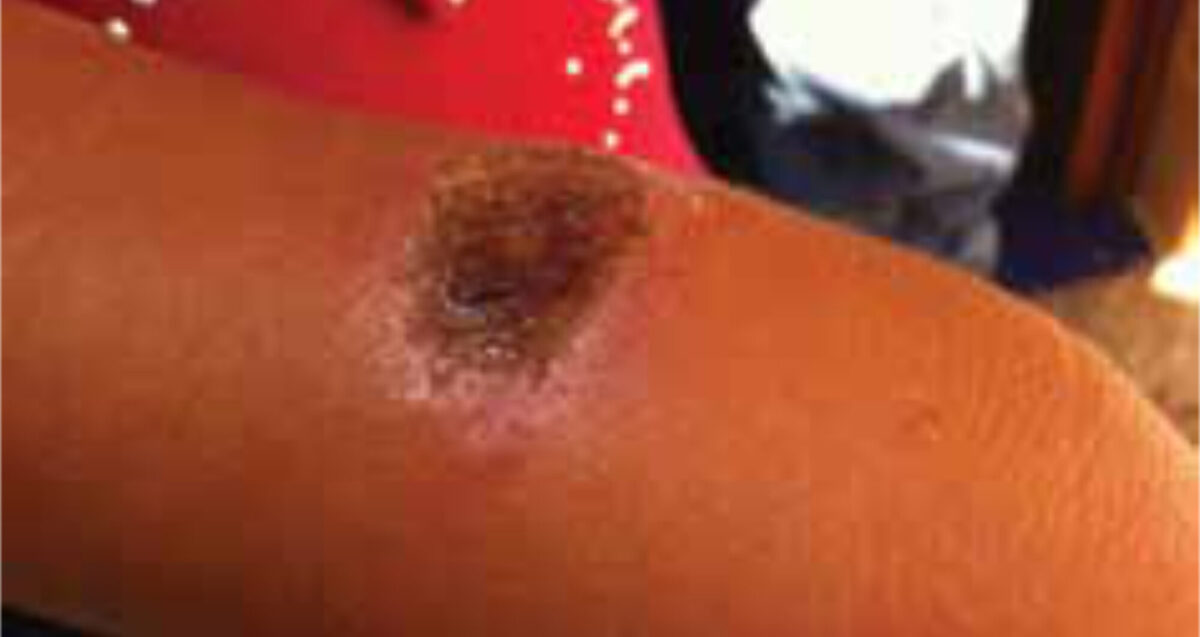
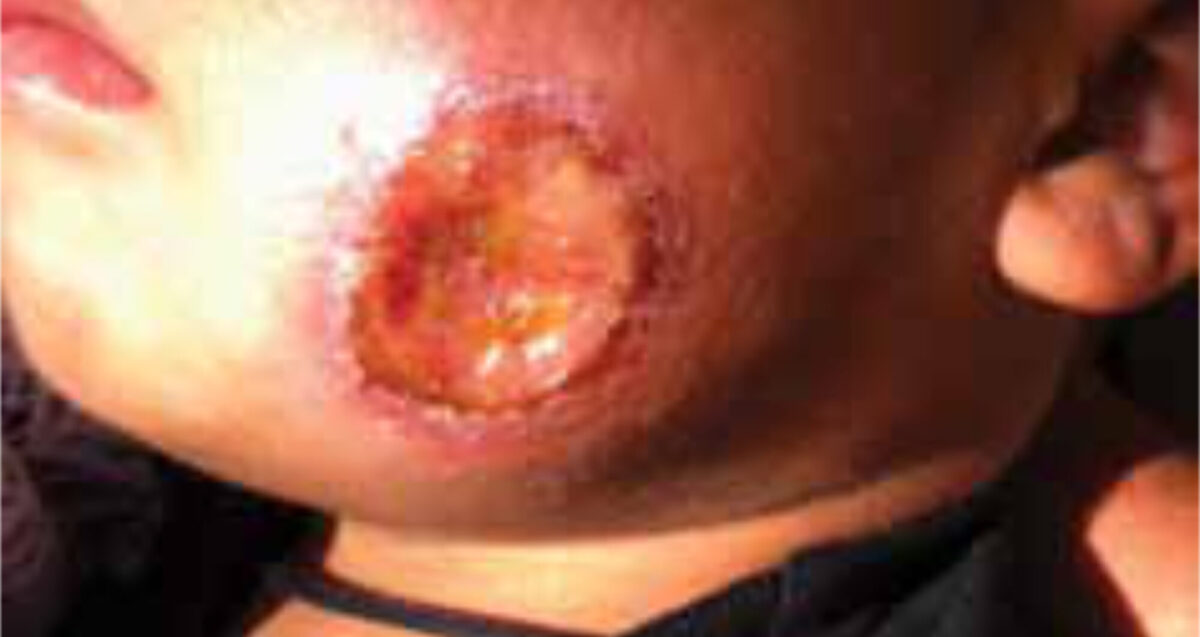
A four-year-old girl and her three-year-old brother, both previously healthy, presented at the outpatient department with painless, non-itching ulcers. The girl had one oval dry ulcer of 2 by 3 cm on the lower right arm (Figures 1, 2), the boy had one round wet ulcer with a diameter of 3 cm on the left cheek and another wet, partly ulcerating nodule of 2 cm on the forehead. Both children also had painless enlarged lymph nodes in the neck, axillae and inguinal regions.
The lesions appeared two months earlier as small nodules that gradually increased in size and finally ulcerated. Treatment of the ulcers with an (unknown) ointment and later with oral penicillin had no effect. Apart from minor discomfort of the lesions, no other complaints were reported. A ten-year-old nephew had had a similar lesion on the ear that healed spontaneously. Besides a slightly raised percentage of eosinophils, the haematology results showed no abnormalities. Scrapings were taken from the floor of the ulcers; no parasites were found. Cutaneous leishmaniasis (CL) was suspected. Because of the negative microscopic findings, CONSULT ONLINE was asked for advice.
Report from the specialists and clinical course
Two dermatologists and one internist-infectious disease physician responded the same day. All were of the opinion that the clinical features fitted in best with CL, whereas deep fungal or bacterial cutaneous infection was considered less likely. To confirm the diagnosis it was advised to take a deep scraping, an aspirate or biopsy from the edge of the ulcer where the Leishmania parasites reside and to examine a Giemsa stained specimen for Leishmanial amastigotes (also called Leishman Donovan bodies). Haematology laboratory results are not of any help. The slightly raised eosinophil count was thought to indicate possible concomitant helminth infection, as protozoan infections usually have no eosinophilia.
It was further suggested that, depending on the Leishmania species, most cases of uncomplicated local CL are self-limiting. However, in our patients prompt treatment was necessary to accelerate healing and to prevent disfiguring scar formation, in particular in the face. First choice are pentavalent antimonials, like PentostamⓇ (sodium stibogluconate) and GlucantimeⓇ (meglumine antimoniate), injected in the edge of the lesion. A second option, pentamidine isethionate is less appropriate, as it can only be administered parenterally and will probably be less effective for the species of this region. Miltefosine, a new oral drug, is costly and not available in this setting.
Because of the lack of a diagnosis and unavailability of anti-leishmanial drugs, the patients were referred to a regional hospital, where infectious diseases should be reported. So far, no feedback has been received.
Discussion
Leishmaniasis is a disease caused by protozoan parasites of the genus Leishmania and transmitted by the bite of female phlebotomine sand flies. About twenty Leishmania species cause human disease. Most species have a reservoir in animals (zoonotic transmission), but some are transmitted from man to man (anthroponotic transmission). The clinical manifestations depend on the species of the parasite and the immune response of the host, and can be divided into three major syndromes: cutaneous (CL), mucocutaneous (MCL) and visceral leishmaniasis (VL), also called kala-azar. [1-4]
The leishmaniases are endemic in 98 countries, mainly in subtropical and tropical areas of all continents except Australia and Oceania. The annual incidence of VL is 0.2-0.4 million and that of CL 0.7-1.2 million cases. [5,6] In Mexico over the period 2004 to 2008 annually 811 cases of CL and 7 of VL were described. [5]
As a result of increasing international travelling in the last decennia cutaneous leishmaniasis especially from the New World, has regularly been encountered in Dutch tourists and military personnel. [7,8]
After infection, papules appear within a few days to weeks. They may develop into nodules that ulcerate and often heal spontaneously within months to years, leaving an atrophic scar. The lesions do not itch and are painless unless secondary bacterial infection develops. Multiple lesions and spread along the lymphatics may occur, resembling sporotrichosis.4 Infection results in lifelong immunity which is not sterile; in case of immunosupression recurrence may occur.
Less than 5 percent of CL cases caused by L. braziliensis progress over time to MCL, with destructive lesions of the nose, oropharynx and larynx. [1,4]
The differential diagnosis of CL includes many diseases, which list can be shortened on the basis of epidemiology, clinical setting and morphology of the lesion. [1,9]
Diagnosis is made by demonstration of parasites in a Giemsa stained aspirate, a scraping from nodules or the edges of the ulcers, or an impression smear from a biopsy. PCR is the most sensitive diagnostic method, but expensive and not available in the resource-poor setting. Culture is laborious, often not available and takes too much time, whereas histopathology may only be of help in experienced hands. [3,4]
As few cases of New World CL may be expected to be self-healing, treatment should be offered particularly in multiple lesions, single lesions on the face or other areas where they cause functional impairment. Another consideration is the risk of MCL; if L. braziliensis is a possibility all patients should be treated. Treatment options include application of heat, cryotherapy, intralesional antimony, ointments with paromomycin or imiquinod, or a combination, and systemic therapy (oral azoles, iv or im antimony, iv or im aminosidine, iv or im amphotericin B [preferably as liposomal formulation], iv or im pentamidine, or oral miltefosine. [2-4]
CL in Mexico and in most other parts of Central America is caused by Leishmania mexicana or Leishmania braziliensis. Uncomplicated lesions with unknown Leishmania species are effectively treated with short course intralesional pentavalent antimony. It is cheap, has no systemic side effects and therefore warrants good compliance. [5,6] However, treatment in the face in young children is not attractive and other options include systemic antimonials and (liposomal) amphotericine B. If the parasite is identified as L. mexicana infection, ketoconazole and miltefosine may also be considered. [6]
At present, no vaccine or chemoprophylactic drug is available. Prevention is aimed at reducing infectious bites of the vector and control measures of the reservoir. [3,4]
Conclusion
In this case report we presented two Mexican children with painless ulcers. Although the diagnosis has not been confirmed, the clinical presentation is very suggestive of cutaneous leishmaniasis and the children were referred to a regional hospital for further management.
The case report gives an example of a common dermatologic disorder with which tropical doctors can be confronted in their daily work. It also gives an example of the burden of neglected tropical diseases in the world’s poorest populations, and shows the importance and need of systematic prevention and control programmes.
References
- Faber W.R., Hay R.J., Naafs B. eds. Imported Skin Diseases. Maarsen; Elsevier: 2006. Chapters 14, 15.
- Ryan T. Cutaneous leishmaniasis (CL). In: Eddlestone M., Davidson R., Brent A., Wilkinson R., eds. Oxford Handbook of Tropical Medicine. Oxford; Oxford University Press: 2010. p. 566-7.
- Ameen M. Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin Exp Dermatol. 2010;35(7):699-705.
- Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581-96.
- Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671.
- World Health Organization. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010. Geneva: WHO; 2010. (Technical report series no. 949)
- Van Thiel P.P., Zeegelaar J.E., van Gool T., Faber W.R., Kager P.A.. Cutaneous leishmaniasis in three Dutch military cohorts following jungle training in Belize. Travel Med Infect Dis. 2011;9(3):153-60.
- Zeegelaar J.E., Steketee W.H., van Thiel P.P., Wetsteyn J.C., Kager P.A., Faber W.R. Changing pattern of imported cutaneous leishmaniasis in the Netherlands. Clin Exp Dermatol. 2005;30(1):1-5.
- Wilson M.E., Chen L.H. Dermatologic infectious diseases in international travelers. Curr Inf Dis Rep 2004;6:54-62.


















































