Main content
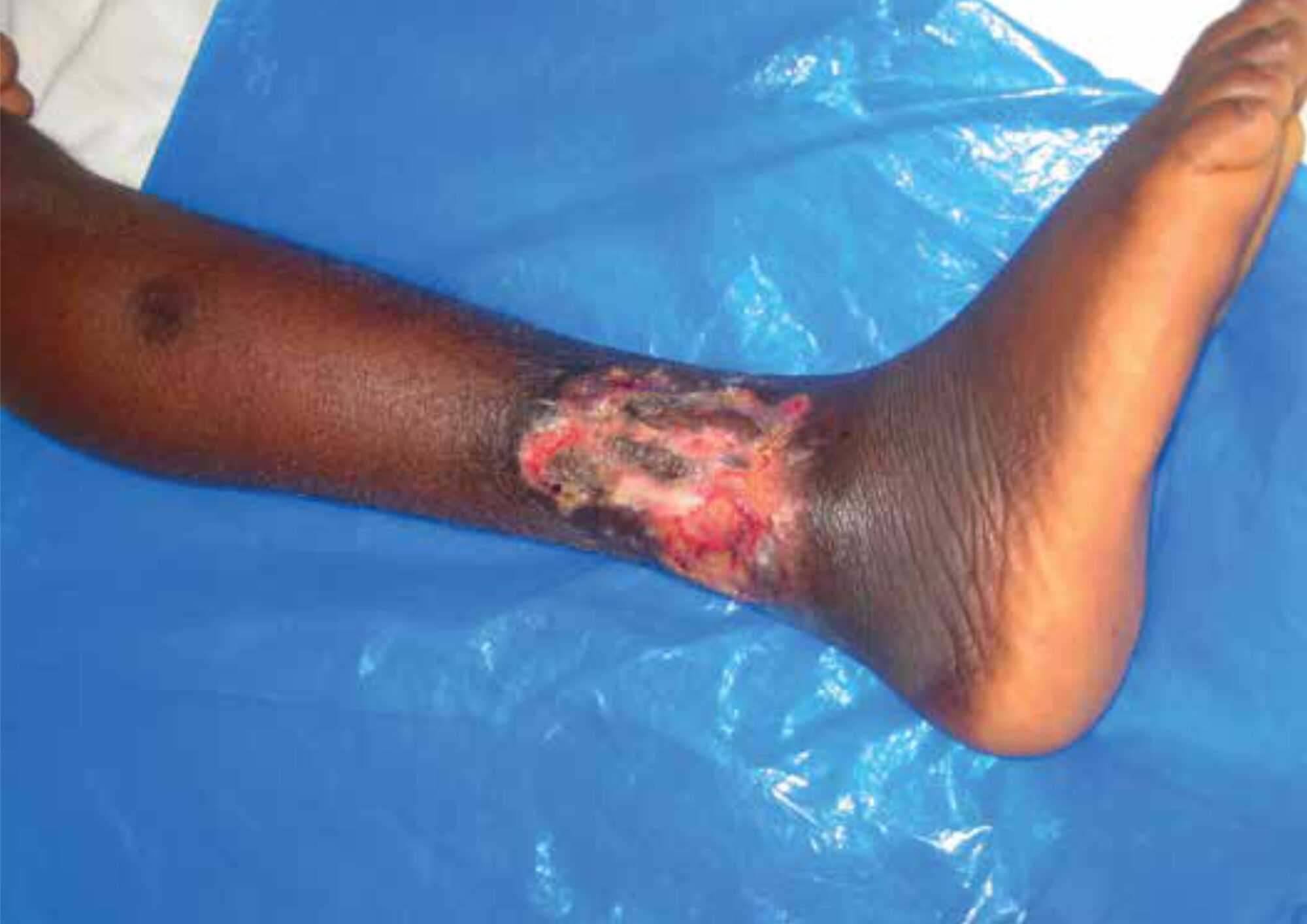
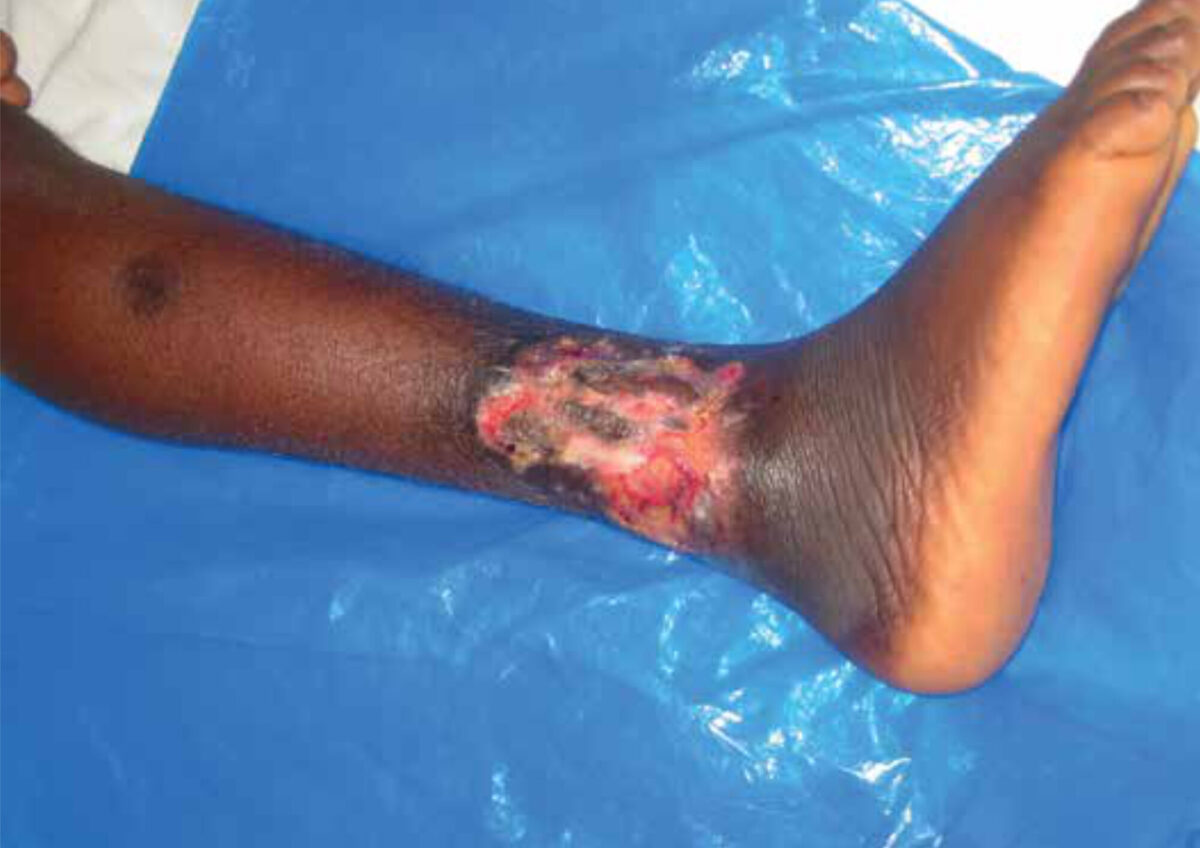
Buruli ulcer (BU) is a disease affecting mostly poor people living in neglected parts of the world. The disease is caused by Mycobacterium ulcerans and leads to nodules or plaques which eventually break down in ulcers with undermined edges (Figure 1). The limbs are the most frequently involved areas; other areas involved are the head, neck, trunk and genital regions. Though prevalent in 33 countries, the disease is endemic mainly in West Africa and is currently showing a steep increase of incidence in Australia. [1,2] Even though it is difficult to measure exact incidence and prevalence numbers of BU, incidence is estimated at 150.8 per 100,000 in Ghana, and prevalence in some villages can be up to 12%. [3] Another study in Ghana in 2014 showed that in a typical hospital the annual burden for treating BU patients amounted to US$ 121,000, with which 75 patients were helped. The financial cost per capita was US$ 1,616. [4] BU frequently leads to long-term disabilities, especially if patients report late to the health care facilities with an advanced stage of the disease. [5] The delay in seeking treatment at the hospital is a major challenge in BU control since it heavily influences the physical problems and stigma caused by the disease. [6]
Diagnosis
Over the past years, diagnostic tools have been improved to help health care workers diagnose BU. Currently, health care workers can send materials to referral laboratory centres to perform staining for acid-fast bacilli and polymerase chain reaction (PCR). Techniques currently under development include the loop-mediated isothermal amplification (LAMP) technique, [7] fluorescent thin layer chromatography (fTLC) for detection of mycolactone, the toxin produced by M. ulcerans, and a point-of-care diagnostic test based on monoclonal antibodies against M. ulcerans. [8] These new diagnostic tests will further reduce the doctor’s delay in diagnosis if available at low cost.
Nevertheless, the main cause of advanced stages of the disease at start of treatment is not the doctor’s delay, but the patient’s delay in seeking treatment at the hospital. This delay is due to financial difficulties, self-treatment and traditional medicine, and the uncertainty about the severity of the skin disease. A practical way to influence such delays is to develop a new diagnostic tool (rapid test) especially for use by the general public living in rural areas. Such a diagnostic tool can inform community member of the most likely diagnosis BU or another skin disease – and the need to report to health care workers.
Antimicrobial treatment
The antimicrobial treatment of BU consists of eight weeks of rifampicin combined with streptomycin intramuscularly. This regimen showed high cure rates in a clinical trial. [9] However, daily intramuscular injections are painful, especially for children, who are most affected. Moreover patients frequently have to travel several hours to access a health facility in order to receive their injection. Prolonged streptomycin administration can also cause oto-, vestibular and/or nephrotoxicity. [10] A recently published randomized clinical trial performed in Ghana and Benin fortunately confirmed that a full oral antimicrobial treatment for early, limited BU with rifampicin and an extended release formulation of clarithromycin is not inferior to the treatment with rifampicin and injected streptomycin. [11]
Based on the findings of this trial, the WHO Technical Advisory Group decided to replace rifampicin and streptomycin by an oral regimen of rifampicin and clarithromycin as the advice for standard treatment for BU. Yet, this advice poses a challenge to national programs and local services in terms of compliance and prevention of misuse of the drugs.
Earlier research on treatment compliance in BU showed poor results during the eight weeks of treatment with rifampicin and streptomycin; in one centre in Ghana, only 46% of the patients completed antimicrobial therapy. [12] Another challenge is to prevent misuse of the new regimen. Clarithromycin can be used for many different infectious diseases and especially its potential in the treatment of respiratory tract infections is liable to lead to misuse of this drug by local trading. This drains the resources for BU treatment and will contribute to development of resistance to clarithromycin and other macrolides in the community. [13]
Wound care
The poor quality of wound care is directly related to serious challenges in the management of BU. Despite the antimicrobial treatment, disabilities are still common, healing takes too much time (median from 18 to 21 weeks), and long hospital stays are still frequently needed and include painful changes of wound dressings. The limited attention given to wound care is illustrated by our previous study on BU wound care in Ghana and Benin which showed a variety in wound care practices among health care personnel and health care facilities; adherence to the WHO guidelines was found to be low. [14] Wound care frequently is accompanied by pain, which is mainly caused by removal of the gauze sticking to the wound without access to adequate pain medication. [15]
Improved wound care has the potential to prevent disabilities and has a direct impact on healing time, length of hospital stays, and long-term consequences of the disease such as contractures.
This need for improved wound care is not unique to BU. The disease burden of skin diseases in general is high in areas where BU is endemic. Patients present with burn wounds, sickle cell and diabetic ulcers or other skin lesions such as those caused by cutaneous leishmaniasis, leprosy or yaws.
Summary
Case detection, community education, and wound care as applied in Buruli ulcer management are examples of activities which can be designed to target multiple neglected skin diseases. Such integration of neglected tropical skin disease activities allows for optimal use of limited resources.
References
- World Health Organization. Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected diseases 2015. Geneva: World Health Organization/Department of Control of Neglected Tropical Diseases; 2015. 191 p. Available from: https://ww.who.int/neglected_diseases/9789241564861/en/.
- Carson C, Lavender CJ, Handasyde KA, et al. Potential wildlife sentinels for monitoring the endemic spread of human buruli ulcer in South-East Australia. PLoS Negl Trop Dis. 2014 Jan 30;8(1):13.
- Amofah G, Bonsu F, Tetteh C, et al. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis. 2002 Feb;8(2):167-70.
- Asare KH, Aikins M. Health facility cost of buruli ulcer wound treatment in Ghana: a case study. Value Heal Reg Issues. 2014 Sep;4:14-8.
- Barogui Y, Johnson RC, van der Werf TS, et al. Functional limitations after surgical or antibiotic treatment for buruli ulcer in Benin. Am J Trop Med Hyg. 2009 Jul;81(1):82-7.
- de Zeeuw J, Omansen TF, Douwstra M, et al. Persisting social participation restrictions among former buruli ulcer patients in Ghana and Benin. PLoS Negl Trop Dis. 2014 Nov;8(11). DOI:10.1371/journal.pntd.0003303.
- Beissner M, Phillips RO, Battke F, et al. Loop-mediated isothermal amplification for laboratory confirmation of buruli ulcer disease. Towards a point-of-care test. PLoS Negl Trop Dis. 2015 Nov;9(11). DOI:10.1371/journal.pntd.0004219.
- Sakyi SA, Aboagye SY, Darko Otchere I, et al. Clinical and laboratory diagnosis of buruli ulcer disease: a systematic review. Can J Infect Dis Med Microbiol. 2016;2016. DOI:10.1155/2016/5310718.
- Nienhuis WA, Stienstra Y, Thompson WA, et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet. 2010 Feb 20:375 (9715):664-72.
- Klis S, Stienstra Y, Phillips RO, et al. Long term streptomycin toxicity in the treatment of buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis. 2014 Mar;8(3). DOI:10.1371/journal.pntd.0002739.
- Phillips, R.O., Robert J, Abass KM, et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet. 2020 Mar; in press.
- Klis S, Kingma RA, Tuah W, et al. Compliance with antimicrobial therapy for buruli ulcer. Antimicrob Agents Chemother. 2014 Oct;58(10):6340.
- George DP. The Macrolide Antibiotic Renaissance. Br J Pharmacol. 2017 Jun. DOI:10.1111/bph.13936.
- Velding K, Klis SA, Abass KM, et al. Wound care in buruli ulcer disease in Ghana and Benin. Am J Trop Med Hyg. 2014 Aug;91(2):313-8.
- de Zeeuw J, Alferink M, Barogui YT, et al. Assessment and treatment of pain during treatment of buruli ulcer. PLoS Negl Trop Dis. 2015 Sep;9(9). DOI:10.1371/journal.pntd.0004076.