Main content
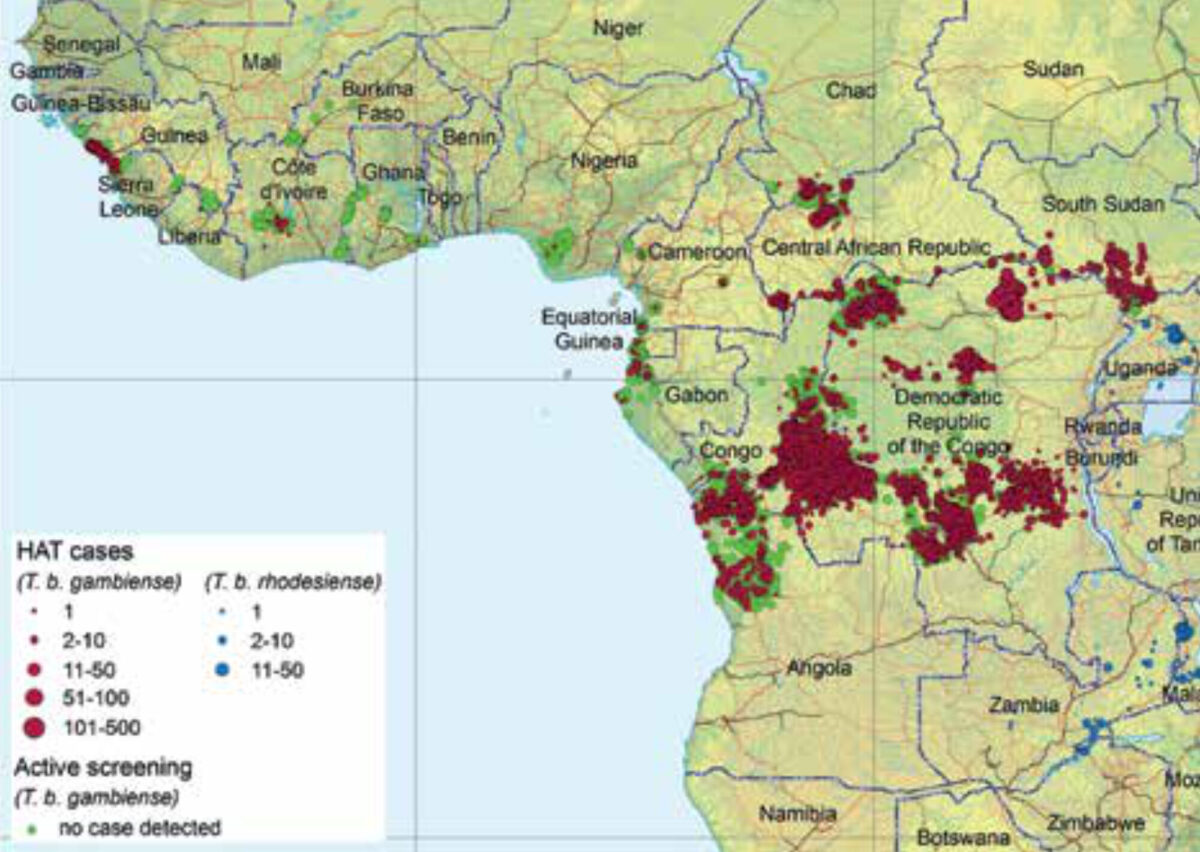
Sleeping sickness or human African trypanosomiasis (HAT) is a parasitic disease present in sub-Saharan Africa caused by two subspecies of the kinetoplastid protozoan Trypanosoma brucei (T.b.). T.b. gambiense is prevalent in Central and West Africa whereas T.b. rhodesiense is present in Eastern and Southern Africa (Figure 1).[1] Other T.b. subspecies are only infective to animals. Trypanosomes show a characteristic movement in vivo due to the presence of an undulating membrane and a trailing flagellum.[1] T.b. is mainly transmitted through the bite of several species of tsetse fly, which are found in distinctive habitats, mainly riverine forests and savannah shrublands.[2] Other transmission modes include mother to child during pregnancy and by blood transfusion or sexual contact, but these are much less frequent or are subject to controversy.
T.b. gambiense is most prevalent, has a higher geographical spread, and evolves chronically over months to years. Its main reservoir is humans, although it has been detected in wild and domestic animals. T.b. rhodesiense evolves acutely over weeks to months, is mainly zoonotic (cattle and game), and humans are accidental hosts. The clinical manifestations of T.b. rhodesiense are more severe, with higher parasitaemia and quicker clinical progression; the heart is commonly affected (pericarditis, myocarditis).
Clinical features
After inoculation through the skin, the clinical presentation of trypanosomiasis is through haemolymphatic spread with rather unspecific symptoms of irregular long-lasting fever, weakness, headache, and weight loss, and may show characteristic lymph node enlargement in posterior cervical angle (Winterbottom’s sign). The sleep-wake rhythm may be disrupted before progression to meningoencephalitic infection, with neuropsychiatric symptoms of advanced disease, including tremor, reappearance of archaic reflexes (palm-chin), walking difficulty, extrapyramidal syndrome, and a frontal syndrome including aggressivity, disinhibition, depression, or apathy. If untreated, this almost always progresses to coma, cachexia, and death.[4] Recently, healthy carriers have been detected, but their role in disease transmission is unknown.[5] Other research has described trypanosomes in the skin, but the clinical relevance is yet to be determined.[6]
History
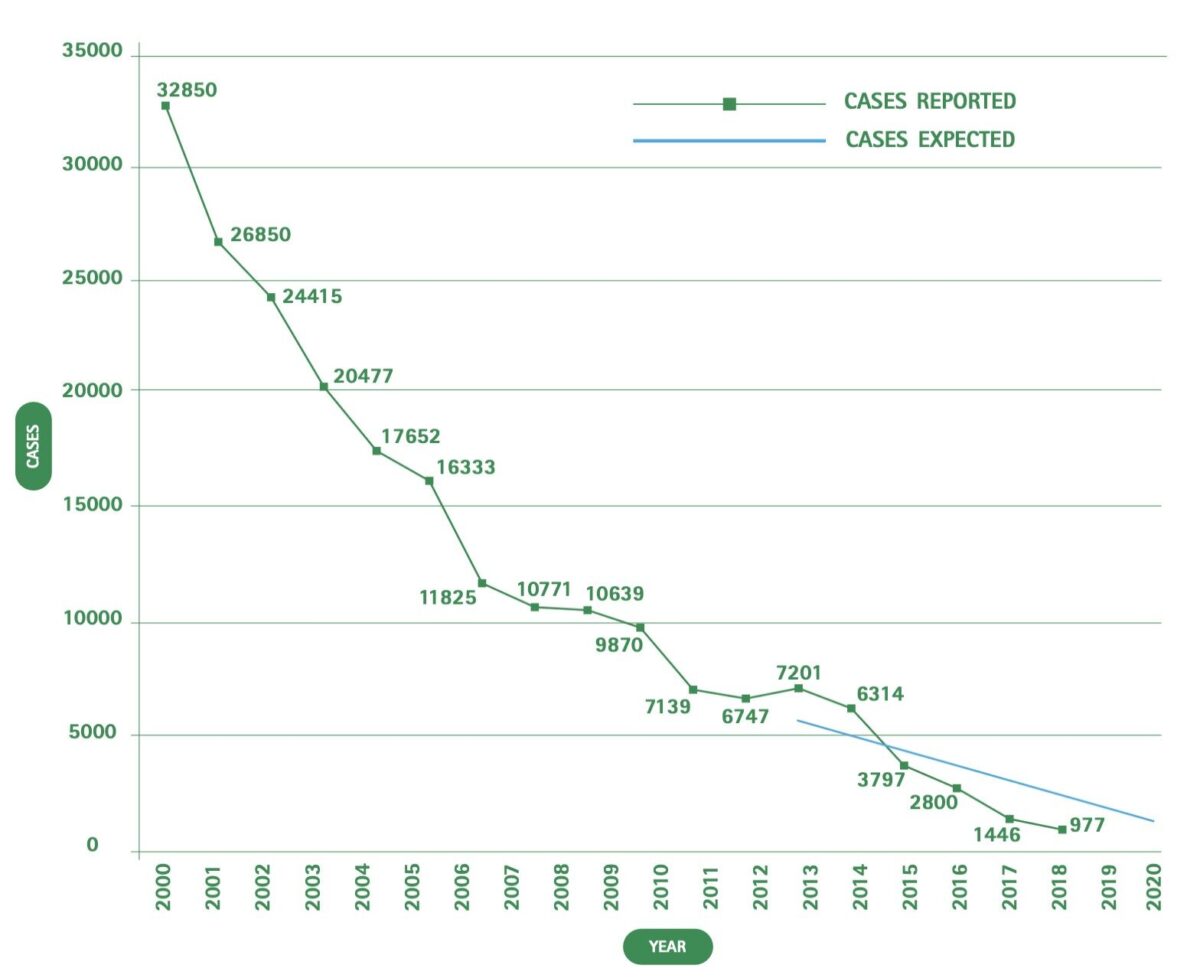
After widespread epidemics in the early twentieth century, there was a steep decrease in the number of cases by the end of the colonial period, with the lowest number by the 1960s. This was related to the intensification of active case detection activities that, for certain countries during the 1940s and 1950s, included systematic chemoprophylaxis with pentamidine and a generally strongly organized vertical control model, which remains largely unchanged today. With the independence of endemic countries, other issues took priority and the colonial health systems were disrupted. It took time for the national governments to replace them, partly due to a widespread lack of trained health professionals. As a consequence, there was an increase in the number of cases, with a peak of detected cases in 1998 (almost 38,000).
Since then, a concerted effort on the part of the World Health Organisation (WHO), the national control programmes, bilateral cooperation (especially Belgian), drug producers (Sanofi and Bayer), and international NGOs such as Médecins Sans Frontières/ Doctors Without Borders (MSF) has led to a steep reduction in the number of cases. In 2012, the WHO defined a roadmap with specific targets for neglected disease elimination. International partners, including the Bill & Melinda Gates Foundation, UK AID/DFID, and the Drugs for Neglected Diseases initiative (DNDi), among other partners supporting HAT elimination, signed the London Declaration on Neglected Tropical Diseases (NTDs), improving international partnership under the WHO coordination towards the roadmap goals for HAT and other NTDs.[7]
In 2018 for the first time the total number of reported cases worldwide was less than 1,000 (Figure 2). Of these, 97.5% were caused by T.b. gambiense. The country with the highest prevalence is the Democratic Republic of Congo (DRC). While sleeping sickness used to be endemic in 36 countries, during the last five years only 14 countries detected at least one case of T.b. gambiense and only five countries detected at least one case of T.b. rhodesiense.[8]
Case detection
Two case-detection strategies have been used since the 1920s: active case search, involving the deployment of mobile teams in endemic areas to examine the entire available population, and passive case search, which is conducted in specially equipped and staffed health structures where suspected symptomatic patients are examined. The main advantage of the active case search strategy is finding cases in initial development of the disease. However, as the number of cases rapidly diminishes, the classic 8- to 10-person mobile teams become too costly per case detected. In an attempt to address this issue, alternative light ‘mini’ mobile teams have been tested and deployed in the DRC. In most endemic areas, passive case detection and treatment remains concentrated in specialized units, separated from the mainstream primary health care system.
Diagnosis
Disease diagnosis relies on a complex algorithm, with serological screening as the first step followed by microscopic examination of fresh samples to detect motile parasites in blood, lymph and, if positive, further examination of cerebrospinal fluid to determine invasion of the central nervous system. Other tests, such as trypanolysis,[9] detecting human antibodies that lyse cloned trypanosomes expressing specific surface glycoproteins, and several DNA/RNA detection tests need highly specialized laboratory structures and are unsuitable for quick field diagnosis, but rather for confirmation of suspected cases or for surveillance purposes.[10]
Serological screening was dependent on the card agglutination trypanosomiasis test (CATT) for a long time. However, this test requires cold chain for optimal performance and is produced in packs of 50 tests. This format is adapted to mobile teams, which perform a series of tests in community interventions. The recently developed rapid diagnostic tests, however, are oriented towards passive case searching at health care structures as they are individually packed and thermostable.
Treatment
For a long time, intravenous melarsoprol was the only treatment for the advanced stage of the disease, a very toxic arsenic derivative developed in 1949.[11] Even now, it remains the reference treatment for advanced T.b. rhodesiense infection. In 1981, the first HAT patient was treated with eflornithine, a much less toxic drug with a very short plasmatic half-life that requires four intravenous infusions per day for two weeks. Its use was thus confined to specialized teams with adequate nursing capacity, most often in general reference hospitals. In 2009, nifurtimox eflornithine combination therapy (NECT) was made available. By adding nifurtimox tablets for ten days, the administration of eflornithine could be reduced to twice a day for seven days,[12] but this remained restricted to a hospital setting (Table 1).
By the end of 2018, fexinidazole, a new, oral-only drug developed by the DNDi[13] was registered in the DRC for the treatment of T.b. gambiense HAT. Fexinidazole will allow the treatment of both early and advanced stage HAT under close supervision in peripheral health structures once staff have been trained, with the exception of those patients with severe neurological or psychiatric symptoms who will still need to be hospitalized and treated with NECT. Specific training of trainers has been conducted by the WHO. This will be followed by an extended training programme targeting 250 hospitals and health centres in all T.b. gambiense endemic countries in 2020 to facilitate access to this simplified treatment and contribute towards treatment availability in areas where patients live. A DNDi study on fexinidazole in T.b. rhodesiense infection is on-going.
A new chemical entity, single oral dose acoziborole, is in a phase 3 clinical trial, in which all study participants have been treated and are in the 18-month follow-up phase. The study is expected to be completed by September 2020. This latest drug in development may be a definitive game changer,[14] contributing to disease elimination as targeted by the WHO roadmap for 2030, possibly allowing for a simplified test and treat strategy.
| HAT STAGE | DRUG | YEAR INTRODUCED | INDICATION | USE |
|---|---|---|---|---|
| EARLY | Suramine | 1917 | T.b. rhodesiense | 5 intramuscular injections 5 days (total duration) 21 days) |
| Pentamidine | 1940 | T.b. gambiense T.b. rhodesiense (2nd line) | Intramuscular injection once a day 7-10 days | |
| ADVANCED | Melarsoprol | 1949 | T.b. rhodesiense T.b. gambiense (3rd line rescue) | Slow intravenous infusion once a day for 10 days |
| Eflornithine | 1981 | T.b. gambiense | 4 intravenous infusions/day for 14 days. | |
| NECT | 2009 | T.b. gambiense (2nd line) | 7 days eflornithine (2 infusions/day) and 10 days oral nifurtimox. First line for severe neuropsychiatric advanced disease | |
| BOTH | Fexinidazole | 2019 | T.b. gambiense (1st line) T.b. rhodesiense (in phase III trial) | 10 days once a day oral treatment. First line except for severe neuropsychiatric advanced disease |
| Acoziborole | 2022 | T.b. gambiense (in Phase III trial) | Single oral dose |
Summary
HAT is at the verge of elimination due to a collective case detection and treatment effort from many stakeholders, coordinated by the WHO and led by the national control programmes in the field. Because of rigorous research carried out in endemic areas under difficult conditions, the management of HAT has changed considerably over the years with old, toxic and intravenous drugs being replaced by patient-friendly regimens with safe, oral short-course drugs.
The management of hat has changed considerably over the years with old, toxic and intravenous drugs being replaced by patient-friendly regimens with safe, oral short- course drugs
References
- Cayla M, Rojas F, Silvester E, et al. African trypanosomes. Parasit Vectors. 2019 Apr;12(1):190. DOI:10.1186/S13071-019-3355-5.
- Attardo GM, Abd-Alla AMM, Acosta-Serrano A, et al. Comparative genomic analysis of six Glossina genomes, vectors of African trypanosomes. Genome Biol. 2019 Sep 2;20(1):187.
- Franco JR, Cecchi G, Priotto G, et al. Monitoring the elimination of human African trypanosomiasis: update to 2016. PLoS Negl Trop Dis. 2018 Dec;12(12). DOI:10.1371/journal.pntd.0006890.
- Kennedy PG. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013 Feb;12(2):186-94-
- Jamonneau V, Ilboudo H, Kaboré J, et al. Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl Trop Dis. 2012 Jun;6(6). DOI:10.1371/journal.pntd.0001691.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016 Sep 22;5. DOI:10.7554/eLife.17716.
- World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. Geneva: World Health Organization; 2012.
- World Health Organization. Global Health Observatory data repository. Human African trypanosomiasis. [updated 2019 Jun 19] [consulted 2020 Feb 9]. Available from: http://apps.who.int/gho/data/node.main.A1635?lang=en.
- Jamonneau V, Bucheton B, Kaboré J, et al. Revisiting the immune trypanolysis test to optimise epidemiological surveillance and control of sleeping sickness in West Africa. PLoS Negl Trop Dis. 2010 Dec;4(12). DOI:10.1371/journal.pntd.0000917.
- Mumba Ngoyi D, Ali Ekangu R, Mumvemba Kodi MF, et al. Performance of parasitological and molecular techniques for the diagnosis and surveillance of gambiense sleeping sickness. PLoS Negl Trop Dis. 2014 Jun;8(6). DOI:10.1371/journal.pntd.0002954.
- Mpanya A, Hendrickx D, Baloji S, et al. From health advice to taboo: community perspectives on the treatment of sleeping sickness in the Democratic Republic of Congo, a qualitative study. PLoS Negl Trop Dis. 2015 Apr;9(4). DOI: 10.1371/journal.pntd.0003686.
- Eperon G, Balasegaram M, Potet J, et al. Treatment options for second-stage gambiense human African trypanosomiasis. Expert Rev Anti Infect Ther. 2014 Nov;12(11):1407-17.
- Mesu VKBK, Kalonji WM, Bardonneau C, et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet. 2018 Jan 13;391(10116):144-154.
- Bottieau E, Clerinx J. Human African Trypanosomiasis: progress and stagnation. Infect Dis Clin North Am. 2019 Mar;33(1):61-77.


















































