Main content
Visceral leishmaniasis (VL or kala-azar) is a neglected tropical disease caused by Leishmania spp., a protozoal parasite that is transmitted by the bite of a Phlebotomine sand fly (Phlebotomus spp. in the Old World, Lutzomyia spp. in the New World). It is estimated that 200,000 to 400,000 new cases of VL occur worldwide each year. The most endemic regions are the Indian subcontinent and East Africa. [1] Over 90% of new cases occur in six countries: Bangladesh, Brazil, Ethiopia, India, South Sudan and Sudan. The global data on incidence in all endemic countries are available. [2] VL runs a fatal course if untreated.
Epidemiology
In endemic areas VL is a disease of childhood, often occurring below the age of five. In outbreaks all ages may be affected. [3] In Asia, Leishmania donovani is the causative parasite; there is no known animal reservoir (anthroponotic transmission). India (Bihar), Bangladesh and Nepal are most endemic. In Africa, VL occurs in Sudan, South Sudan, Ethiopia, Kenya, Uganda and Somalia; here also L. donovani is the parasite involved, but it is genetically very different from that found in Asia. [4] The transmission is mixed anthroponotic and zoonotic, although the exact animal reservoir and its contribution has not been identified.
All countries in the Mediterranean basin are endemic. Here, and in South and Central America, VL is caused by L. infantum, and dogs are known reservoirs. In endemic and non-endemic countries, VL may occur among travellers, ecotourists, immigrants or military personnel. There is a risk that imported cases in endemic areas may introduce new strains. [5] This may be particularly relevant given the mass migration of people from VL-endemic countries in the Middle East, East Africa and Asia that started in 2015. Immunosuppression is a major risk factor for VL. This includes patients with HIV/AIDS, or those who have other conditions associated with immunosuppression such as haematological malignancies. [6] Not all infections lead to clinical disease; the number of asymptomatic or subclinical infections usually outnumber the clinical cases by a ratio of 1:5.

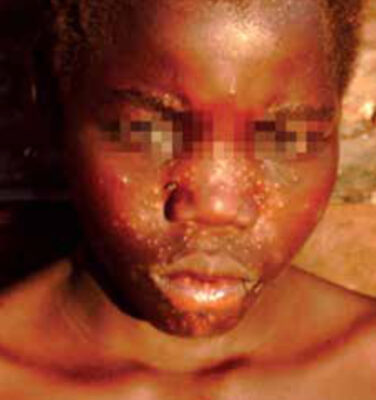
leishmaniasis (PKDL), with papules mainly in
the face as the result of inflammation around
leishmania parasites that persist after VL.
Clinical presentation
The incubation period is 2-6 months. The patient usually presents with fever >3 weeks; in endemic areas there is often a history of fever not responding to antimalarials. Weight loss and abdominal left quadrant upper pain caused by spleen enlargement are common symptoms; on physical examination fever, hepatosplenomegaly and malnutrition are commonly found (Figure 1). In HIV co-infected VL patients, the clinical presentation is similar but unusual presentations occur, such as dermal lesions.
Post-kala-azar dermal leishmaniasis (pkdl)
PKDL is characterized by a skin rash that may develop after treatment of VL (Figure 2). It is most common in areas where L. donovani is the causative agent of VL (Africa, Asia). Most patients are well except for the rash. There are important regional differences. While in Africa a predominantly papular rash occurs in up to 50% of patients after VL treatment, in Asia the rash is often macular and occurs only after 1-3 years after VL, in up to 20% of patients. It is thought that PKDL patients may play an important role in outbreaks of VL because leishmania parasites may be found in the skin lesions and sand flies may become infected after feeding on PKDL patients.
Images of PKDL can be found in The Post Kala-azar Dermal Leishmaniasis (PKDL) Atlas: A Manual for Health Workers, published by the World Health Organization (WHO). [7]
Laboratory and radiological diagnosis
Laboratory tests typically show pancytopenia. Other features include low albumin and high gamma globulin levels. Ultrasound examination may be useful for differential diagnosis and to confirm hepatosplenomegaly and lymphadenopathy.
Parasitological
Parasites may be demonstrated by classical microscopic examination of aspirates of lymph node, bone marrow or spleen, with increasing sensitivity (approximately 55%, 70% and 95%, respectively). [8] Molecular diagnosis involves detection of leishmanial DNA by PCR that has the highest sensitivity and specificity. qPCR quantifies the parasite load and may be used as a biomarker to monitor treatment. Field adapted formats are being developed.
Serological
Serological tests including direct agglutination test (DAT), immunofluorescence tests or ELISA that take advantage of the high gamma globulin levels (anti-leishmania antibodies). The rK39 rapid diagnostic (RDT) test is easy to use in the field and widely accepted for first line diagnosis in the Indian subcontinent. Other tests detect leishmania antigens in the urine.
Immunological
The leishmanin skin test (LST) measures cell-mediated immunity; killed Leishmania promastigotes are injected intradermally and the skin induration is read after 48-72 hours. The LST is mainly used for screening purposes; a positive test indicates past exposure (clinical or asymptomatic infection).
Treatment
Guidelines for treatment have been published by the WHO according to the strength of available data. [9] Sodium stibogluconate (SSG) has long been the mainstay of treatment; it is given for 30 days by IM or IV injections that are painful. There is concern about side-effects, mainly cardiotoxicity.
In Asia, SSG is no longer effective because of resistance. Single dose AmBisome (liposomal amphotericin B) (SDA) in a dose of 10 mg/kg is the current standard treatment with cure rates of 96-98% in India and Bangladesh. [10]
Globally, miltefosine is the only oral drug available; currently it is given as treatment for PKDL patients for 90 days. [11]
In Africa, combination therapy with stibogluconate and paromomycin for seventeen days is currently the standard regimen, and this has replaced SSG monotherapy given for 30 days. [10]
In the Mediterranean countries and South America, AmBisome (multiple doses) is used as first-line treatment. HIV co-infected patients are treated with AmBisome combined with miltefosine. Because of the immunodeficiency, these patients often relapse, and maintenance treatment (secondary prophylaxis) is needed with injections of an anti-leishmanial drug (e.g. AmBisome) every 3-4 weeks, in addition to antiretroviral therapy. [10]
Current needs
There is a need for new, oral, short course regimens for both VL and PKDL. The Drugs for Neglected Diseases initiative (DNDi), Geneva, (www.dndi.org) is a not-for-profit organisation committed to research and development of new drugs for neglected tropical diseases (NTDs) and has delivered new treatment options in Asia and Africa, including options for HIV-VL co-infected patients. [10]
In addition to new drugs, a better understanding of the pathophysiology of VL and PKDL is needed to identify biomarkers for diagnosis and disease progression and as entry points for immune manipulation.
Control
Clearly, control strategies depend on regional characteristics of the disease.
The South-East Asia region kala-azar elimination program (KAEP) adopted a multi approach strategy aiming to reach the elimination target of less than one kala-azar case per 10,000 people per year by 2015. The program focuses on active case finding of kala-azar using rapid point-of-care diagnostic tests (rK39 strip test) and treatment with SDA with combination therapies as alternative treatments. Sand fly vector control completes the approach. [12] In India, good progress has been made. Increased awareness, less stigma, use of mosquito nets and SDA has resulted in a three-fold reduction of cases and mortality in Bihar alone. However, it is doubtful if the elimination target will reached in 2015. [13] Among threats to the program are the detection and management of PKDL patients, the role of asymptomatic patients, and concerns about quality assurance and resistance of sand flies to DDT. [14,15]
In Africa, there is currently no control strategy, mainly because of lack of political commitment and the unstable situation in South Sudan and Somalia. This is complicated by the lack of information on the sand fly vector regarding biting habits and habitat. [16] Long-lasting insecticidal bed nets were associated with reduced disease incidence in Eastern Sudan. [17] Several animals have been identified as potential reservoirs, but the contribution of each has not been established. Active and passive case finding of VL and PKDL cases may reduce the human reservoir. PKDL patients are a likely reservoir, but currently only chronic and severe cases are treated.
In the Mediterranean area, dogs may be protected by insecticide-containing collars; sick dogs are culled as they do not respond well to treatment. Currently there is no vaccine for human use; dogs can be vaccinated.
Summary
Visceral leishmaniasis continues to be an important neglected tropical disease despite considerable progress in understanding the pathophysiology and improved management. In Asia, the control program seems successful so far, but the role of PKDL and asymptomatic infection needs to be clarified. VL control in Africa is problematic. Vigilance of health authorities in non-endemic areas for VL as an imported disease continues to be appropriate in view of increased migration and travel.
References
- World Health Organization. Leishmaniasis. 2020 Mar 2. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/.
- Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012 May;7(5) DOI:10.1371/journal.pone.0035671.
- Seaman J, Mercer AJ, Sondorp E. The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: course and impact from 1984 to 1994. Int J Epidemiol. 1996 Aug;25(4):862-71. Epub 1996 Aug 1.
- Lukes J, Mauricio IL, Schonian G, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci U S A. 2007 May 29;104(22):9375-80. Epub 2007 May 21.
- Di Muccio T, Scalone A, Bruno A, et al. Epidemiology of imported Leishmaniasis in Italy: implications for a European endemic country. PLoS One. 2015 Jul;10(6). DOI:10.1371/journal.pone.0134885.
- Komitopoulou A, Tzenou T, Baltadakis J, et al. Is leishmaniasis an “unusual suspect” of infection in allogeneic transplantation? Transpl Infect Dis. 2014 Dec 16;16(6):1012-8. Epub 2014 Nov 21. DOI:10.1111/tid.12316.
- WHO/Department of control of neglected tropical diseases. The post Kala-azar dermal leishmaniasis (PKDL) atlas: a manual for health workers. 2012. 214 p. Available from: http://apps.who.int/iris/bitstream/10665/101164/1/9789241504102_eng.pdf.
- Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2001 Apr;95 Suppl 1:S27-58. Epub 2001 May 24.
- World Health Organization. Control of the Leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010. Geneva. World Health Organization. 2010. 202 p. World Technical Report Series No. 949.
- Alves F, Bilbe G, Blesson S, et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev. 2018 Aug;31(4). DOI:10.1128/CMR.00048-18.
- Sundar S, Sinha P, Jha TK, et al. Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial. Trop Med Int Health. 2013 Jan;18(1):96-100. Epub 2012 Nov 8. DOI:10.1111/tmi.12015.
- World Health Organization, Regional Office for South-East Asia. Regional strategic framework for elimination of kala azar from the South-East Asia Region (2005-2015). WHO Regional Office for South-East Asia. 2005. 22 p. Available from: https://apps.who.int/iris/handle/10665/205825.
- Cousins S. India makes good progress in combating kala-azar. Lancet. 2015 May;385(9979):1716. DOI:10.1016/S0140-6736(15) 60877-7.
- Coleman M, Foster GM, Deb R, et al. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci US A. 2015 Jul;112(28):8573-8. DOI:10.1073/pnas.1507782112.
- Le Rutte EA, Zijlstra EE, de Vlas SJ. Post-kala-azar dermal leishmaniasis as a reservoir for visceral leishmaniasis transmission. Trends Parasitol. 2019 Aug;35(8):590-2. Epub 2019 Jun 29 DOI:10.1016/j.pt.2019.06.007.
- Elnaiem DE. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J Vector. 2011 Mar;36 Suppl 1:S23-31. DOI:10.1111/j.1948-7134.2011.00109.Χ.
- Ritmeijer K, Davies C, van Zorge R, et al. Evaluation of a mass distribution programme for fine-mesh impregnated bednets against visceral leishmaniasis in eastern Sudan. Trop Med Int Health. 2007 Mar;12(3):404-14. Epub 2007 Feb 23.


















































