Main content
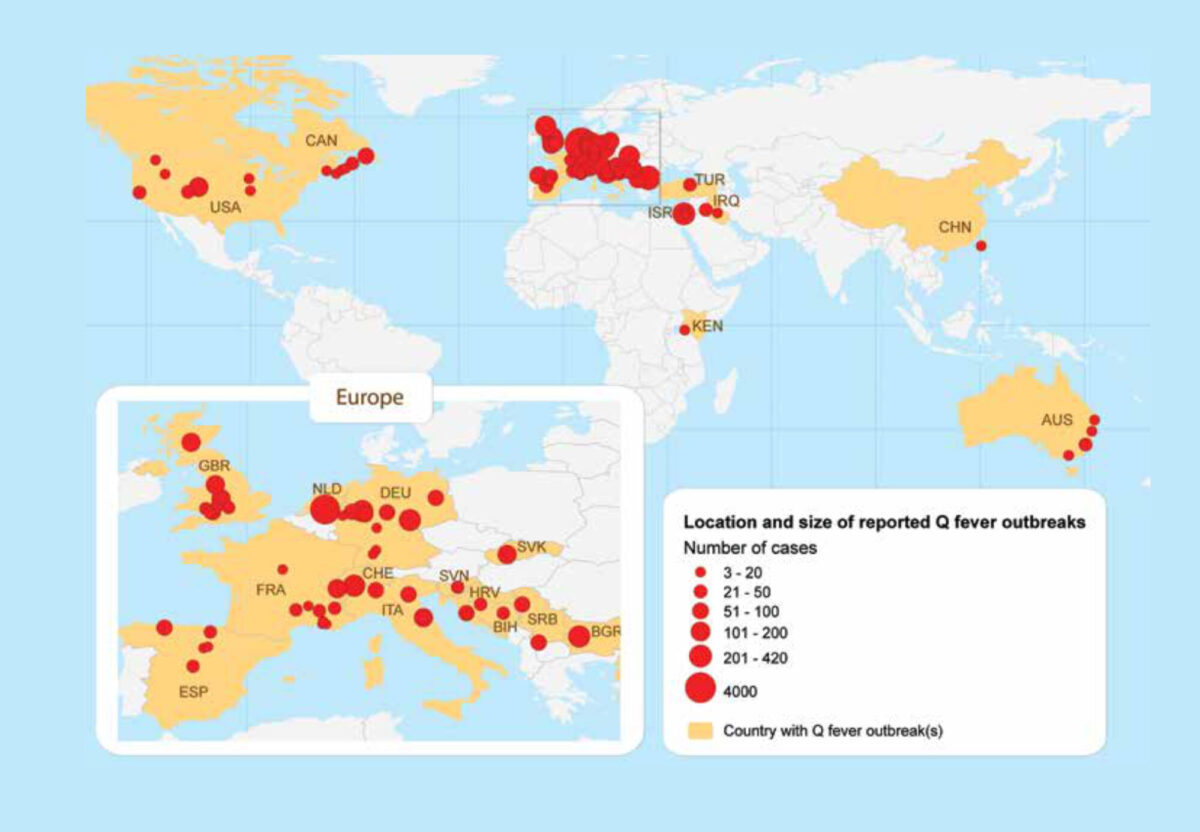
Q fever is a bacterial zoonotic disease that was first described in Australia, in 1937. Regular outbreaks among humans have since been described in Europe, North America, the Middle East and Australia (Figure 1). Studies among patients with febrile illness or pneumonia, and seroprevalence studies among humans and animals have demonstrated the worldwide occurrence of the causative bacterium Coxiella burnetii. In the large majority of outbreaks, small ruminants (sheep or goats) were identified as the source but other animals have been implicated, including cattle, domestic cats, dogs, pigeons, and wild rabbits. New Zealand is the only country in the world where studies have been done, but where the bacterium and the disease were found to be absent. From 2007 to 2010, the Netherlands faced the largest Q fever epidemic that was ever described and this has placed Q fever again high on the agenda as an emerging, or re-emerging, zoonosis. The present paper focuses on the extent of the problem of Q fever in sub-Saharan Africa.
Causes of fever in Africa
Until recently, the paradigm in much of sub-Saharan Africa was that malaria is the overwhelming cause of fever and that patients with febrile illness must receive presumptive treatment with artemisinin-based combination therapy (ACT). However, the epidemiology of malaria is changing with substantial decline in disease prevalence and incidence in a number of African countries. For the African continent it was estimated that in 2007, there were 656 million fevers among 0-4 year old children, 182 million were likely to have sought treatment in a public sector clinic of which 78 million were likely to have been infected with Plasmodium falciparum.² Therefore, 57% of the 182 million children presenting with fever to government-supported clinics do not have malaria infection, and in some countries, that proportion is greater than 90%. These findings are supported by country-specific studies. For example, in the Gambia, during 22 weeks follow-up of a cohort of 800 children in the 2008 malaria season, only 11% (24/224) of febrile episodes detected were due to malaria.³
The official World Health Organization (WHO) policy is that all suspected cases of malaria should be diagnostically confirmed before treatment, with microscopy or a malaria rapid diagnostic test (RDT). This will allow for the restricted use of ACT for those who actually have malaria. However, the question remains what to do with febrile patients who test negative for malaria. Indiscriminate use of antibiotics must be avoided and there is an urgent need for cheap point-of-care rapid tests to diagnose patients that have a bacterial infection. A study in Tanzania among hospitalised febrile patients showed that 5.0% (24/483) had acute Q fever and 8.4% (38/450) rickettsial infection.⁴ These conditions were not clinically diagnosed in any patient and the most common diagnoses among subsequently identified cases of Q fever and rickettsiosis were malaria and pneumonia. Patients therefore did not receive appropriate treatment.
While acute Q fever may present as febrile illness, pneumonia, or hepatitis, approximately 2% of acute Q fever patients develop chronic Q fever, a very serious condition with endocarditis and vascular infection as the main presentations. In cohort studies in Africa, Q fever accounted for 2-9% of febrile illness hospitalisations,⁵ but also for 1-3% of infective endocarditis cases.⁵
C. burnetii in animals and humans
Data on Q-fever in African countries are limited but it is clear that infection with C. burnetii occurs throughout Africa with seroprevalence of antibodies in humans from 1% to >20%.⁵,⁶ In a study in Ghana, 16.9% of 219 2-year-old children and 8.9% of 158 healthy adults were positive for IgG phase II antibodies against C. burnetii, suggesting previous infection.⁷ It is a remarkable finding from several studies in Africa that seroprevalence of antibodies against C. burnetii is higher among children than among adults, while it is the opposite in studies in Europe. In a study in the Gambia, 63/796 (7.9%) 1-15 year old children tested positive for C. burnetii antibodies.⁸ A veterinary survey among 566 sheep and goats from the same area showed a seroprevalence of antibodies against C. burnetii of 17.5%.⁹ This suggests that there is considerable exposure to C. burnetii in children in rural Africa and that Q fever must be considered as a cause of acute febrile illness. A systematic review showed a small ruminant seroprevalence in Africa ranging from 11-33%.⁵ In ruminants, the infection is often asymptomatic but abortions and reduced reproductive efficiency may occur, with subsequent socioeconomic consequences for humans.⁵,¹⁰
Control measures
Most emerging infectious diseases are zoonoses, and the majority of emerging infectious disease events are caused by bacteria or rickettsiae.¹¹ Q fever can be considered a prototype bacterial zoonotic infectious disease that is hugely underdiagnosed. Improved recognition and individual patient management would require a rapid point-of-care diagnostic test that is currently not available. Preventive measures are also difficult to implement. In many pastoral and mixed crop-livestock systems in sub-Saharan Africa, people live very closely with livestock populations. Livestock play a crucial role in the livelihoods of the majority of Africans providing meat, milk, traction, nutrients for crops (e.g. manure) and cash income. In fact, it has been suggested that ownership of small ruminants and other animals can be a key factor for poor households to jump the poverty line.¹²
Conclusion
Q fever presents an important but underappreciated threat to human and animal health throughout Africa. Children in rural sub-Saharan Africa become exposed to C. burnetii early in life and Q fever, which is difficult to distinguish from malaria, may develop and remain untreated in a considerable proportion of them.
References
- van der Hoek W, Dijkstra F, Schimmer B et al. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 2010;15(12):pii=19520.
- Gething PW, Kirui VC, Alegana VA et al. Estimating the number of paediatric fevers associated with malaria infection presenting to Africa’s public health sector in 2007. PLoS Med 2010;7(7):e1000301.
- Ceesay SJ, Casals-Pascual C, Nwakanma DC et al. Continued decline of malaria in The Gambia with implications for elimination. PLoS One 2010;5(8):e12242.
- Prabhu M, Nicholson WL, Roche AJ et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis 2011;53(4):e8-e15.
- Vanderburg S, Rubach MP, Halliday JE et al. Epidemiology of Coxiella burnetii infection in Africa: a OneHealth systematic review. PLoS Negl Trop Dis 2014;8(4):e2787.
- Tissot-Dupont H, Brouqui P, Faugere B et al. Prevalence of antibodies to Coxiella burnetii, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin Infect Dis 1995;21:1126-33.
- Kobbe R, Kramme S, Kreuels B et al. Q fever in young children, Ghana. Emerg Infect Dis 2008;14:344-6.
- Van der Hoek W, Sarge-Njie R, Herremans T et al. Prevalence of antibodies against Coxiella burnetii (Q fever) in children in The Gambia, West Africa. Trop Med Int Health 2013;18:850-3.
- Klaasen M, Roest HJ, van der Hoek W et al. Coxiella burnetii seroprevalence in small ruminants in The Gambia. PLoS One 2014;9:e85424.
- Perry BD, Grace D, Sones K. Current drivers and future directions of global livestock disease dynamics. Proc Natl Acad Sci USA 2013;110:20871-7.
- Jones KE, Patel NG, Levy MA et al. Global trends in emerging infectious diseases. Nature 2008;451:990-3.
- Peacock C. Goats—A pathway out of poverty. Small Ruminant Res 2005;60:179-86.



















































