Main content
Setting
This case is set in Kikori District Hospital on the southwest peninsula of Papua New Guinea (PNG). The hospital is located in the Gulf province on the banks of the Purari River amidst tropical forest. The district hospital has 80 beds and very limited diagnostic tools. There is no access to laboratory tests, other than those used to diagnose HIV, syphilis, tuberculosis and malaria. Ultrasound is available, and an X-ray is about to be installed.
PNG has one of the highest tuberculosis rates in the world, with an estimated incidence of 400 per 100,000 inhabitants.¹ In the Gulf province, the incidence is about three times higher: 1290 per 100,000 patients in 2012.² Furthermore, there is an increasing incidence of drug-resistant strains. MDR-TB is found in 2.7% of all newly diagnosed TB patients and in 19% of patients requiring retreatment.²
Apart from TB, PNG has a high incidence of malaria and other tropical infectious illnesses. Significant health issues occur due to malnutrition and poor hygiene. Furthermore, physical violence is rampant partly associated with alcohol and drug use (marihuana and chewed betel nut).
Case
A 25-year old male patient presented at the out-patient clinic with a lesion to the left foot. The wound had started five weeks earlier and was progressive, with the development of two satellite lesions. The patient was unsure how the lesion had commenced. The history mentioned water contact but no recent trauma. Apart from the lesion, the patient was healthy and did not suffer any other symptoms. He had never been diagnosed with diabetes and his vascular status was unknown. He did not use any medication but consumed large quantities of tobacco, alcohol, marihuana and betel nut. He was unemployed. Family history included an aunt with pulmonary tuberculosis.
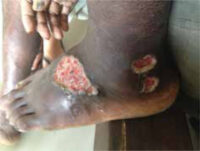
On physical examination, a large ulcer and two smaller lesions with undermined edges and oedema were seen on the dorsolateral side of the left foot (Figure 1). There was no pain, odour or purulent exudate. There was no fever. On auscultation of the lungs, normal sounds were heard. Several small lymph nodes were palpable bilaterally in the supraclavicular region.
Specialist advice
A diagnosis of Buruli ulcer was made and the attending physician suggested starting rifampicin and streptomycin. However, he was concerned his suggested therapy would lead to drug resistance in this patient, whose a priori chance of having tuberculosis was high (taking into account the high incidence of TB in the region as well as the family history). Our specialists were consulted regarding two specific questions. Was a Buruli ulcer the most probable diagnosis? And would they suggest treating this patient with a regular tuberculosis regimen instead of the suggested specific antimicrobial therapy for Buruli ulcer?
Within 24 hours, three specialists (an infectiologist and two dermatologists) had responded to the enquiry. The dermatologists were unanimous in confirming the diagnosis of a Buruli ulcer. For further validation of the diagnosis, they suggested taking a smear to detect for acid-fast bacilli (AFB) to demonstrate the presence of mycobacteria. Regarding the treatment of this patient, they were less unanimous. One dermatologist recognized the concern of inducing drug resistance and advised starting the patient on a regular tuberculosis regimen (including streptomycin). The other dermatologist was not convinced of the indication for a full course of antituberculous therapy, based on the symptoms of the patient. He advised starting the patient on rifampicin/streptomycin or rifampicin/clarithromycin to treat the Buruli ulcer accordingly and to disregard a possible concurrent tuberculosis infection.
The infectiologist offered different advice altogether. Although the ulcer could be caused by M. ulcerans, this diagnosis would not explain the lymphadenopathy. He proposed regarding the ulcers and lymphadenopathy as manifestations of the same illness and treating the patient for tuberculosis. He recognized the risk of inducing drug resistance by treating for Buruli ulcer while the patient could actually have tuberculosis.
He suggested further examination including chest X-ray and FNA of the lymph nodes. As this was not possible in Kikori district hospital, the advice was to start quadruple therapy for tuberculosis³, adding streptomycin for 4 weeks. Also, the infectiologist suggested testing the patient for concurrent HIV infection, as disseminated TB occurs more often in HIV positive patients. Naturally, in the case of a positive test result, this would warrant treating the patient for HIV.
Follow-up
To confirm the diagnosis, a smear was taken that showed AFBs. Regarding the different treatment recommendations, the physician decided to start the patient on a full-course tuberculosis regimen. There was satisfactory healing tendency. After a week of therapy, including regular wound debridement, the oedema had disappeared and the ulcers no longer featured undermined edges. Thereafter, gradual granulation was seen. The smaller ulcers disappeared and the large ulcer decreased to about 2 cm.
Unfortunately, the patient was not compliant with his therapy and did not report for follow-up. He eventually returned after 22 days of non-compliance. His regimen was resumed, adding the 22 days he missed due to his absence in the initial phase. Thereafter, he will receive 4 months of treatment during the continuation phase.
Evaluation
This case demonstrates that there is no simple answer to the question in the title of this article. Several different treatment strategies were proposed in line with the experience and opinion of the specialists concerned. After weighing all the options, the physician chose to treat with a full course of antituberculous therapy. It is possible, however, that the other proposed therapeutic strategies might also have led to satisfactory results. Similar cases should be evaluated individually, taking into account the incidence of Buruli ulcer, TB and MDR-TB in the region as well as the clinical evidence for either Buruli ulcer or cutaneous TB.
Background: buruli ulcer
A Buruli ulcer is a skin infection caused by Mycobacterium ulcerans.⁴ Following tuberculosis and leprosy, it is the third most common mycobacterial infection worldwide. It occurs mainly in humid, rural and tropical areas where access to health care is limited. Transmission is thought to occur through contaminated water, involving skin trauma or transmission via aquatic insects.⁴
Clinical manifesta-tions and diagnosis
The classic initial manifestation of a Buruli ulcer is a painless nodule, papule or plaque of less than 5 cm in diameter. After several days to weeks, this primary lesion forms an ulcer. Typically, the ulcer is painless, with undermined edges and slow progression. A Buruli ulcer is most frequently located on the limbs. Systemic symptoms can be found in the occurrence of secondary bacterial infections. Infection can progress to deeper tissues (tendons, bones) and can spread contiguously or haematogenously.
Treatment
In up to 33% of patients, spontaneous healing occurs, although this can take months and may lead to scarring, retraction and contractions, thereby giving rise to functional disability. Treatment consists of systemic antibiotic therapy, most commonly consisting of rifampicin (10 mg/kg orally 1dd) and streptomycin (15 mg/kg IM 1dd), during 4 to 8 weeks (depending on the size of the ulcer).⁴,⁶ Drug therapy can be complemented by surgical debridement. Prevention consists of limiting exposure to contaminated water sources.
Background: cutaneous tuberculosis
Cutaneous tuberculosis is rare and accounts for less than 1-2% of all tuberculosis cases.⁷ There are three different classifications based on pathogenesis: direct inoculation from an exogenous source (e.g. tuberculous chancre), from an endogenous source (e.g. scrofuloderma) and haematogenous tuberculosis (e.g. lupus vulgaris).⁸
Clinical features
The diagnosis of cutaneous TB is difficult, as the clinical features vary greatly. It can present with small warty lesions, papules or plaques, but can also feature ulcers.⁸,⁹ Compared to Buruli ulcer, the lesion is more likely to be painful and systemic symptoms are found more often. Microbiological confirmation includes PCR and the gold standard of isolating Mycobacterium tuberculosis in culture.⁷ However, this can be difficult due to a low bacillary load.¹⁰,¹¹
Treatment
Similar to MOTT (Mycobacterium other than Tuberculosis) infections, cutaneous tuberculosis can resolve spontaneously, leaving a scar. Treatment relies on quadruple therapy as used in pulmonary TB (initial phase of isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin during 2 months, followed by a continuation phase of 4 months with isoniazid and rifampicin).³,⁷
Background: drug resistant tb
There are two different types of drug resistant TB. Multi-drug resistant (MDR) TB is resistant to both of the first line drugs, isoniazid and rifampicin. Extensively drug-resistant (XDR) TB-strains have an additional resistance to fluoroquinolones and second-line injectable agents (amikacin, capreomycin, kanamycin).¹² Of all new cases of TB worldwide, 3.3% are MDR-TB. In patients who have previously received tuberculostatic drugs, this proportion is even higher (20%).¹²
Causes of multidrug resistance include higher bacillary load, drug shortages, inappropriate drug levels, poor compliance (due to behavioural pattern or side effects) and malabsorption of medication due to associated diarrhoea.
Diagnosis of MDR-TB is difficult, and the majority of cases is not correctly identified.¹²,¹³ Laboratory facilities for TB culture and drug susceptibility testing are limited. Extrapulmonary (including cutaneous) TB is paucibacillary and therefore difficult to recover on culture.¹⁰,¹¹ The pitfall is that a lack of therapeutic response in MDR-TB can be regarded as suggestive of an alternative diagnosis. Better diagnostics for rapid, reliable diagnosis of MDR-TB are necessary, as earlier diagnosis will lead to more effective treatment. Currently, both GeneXpert MTB/RIF (Xpert) and Genotype MTBDRplus (DRplus) have been endorsed by WHO for rapid diagnosis of MDR-TB using molecular techniques.¹⁴,¹⁵ The former has high sensitivity and specificity and can be carried out in 2 hours.
Treatment consists of at least five drugs during an 8-month intensive phase, followed by a continuation phase of four oral drugs for a total minimal duration of 20 months.¹³ Challenges include prolonged hospitalization, nephro- and ototoxicity and poor treatment adherence. Furthermore, treatment for MDR-TB and XDR-TB is 25 times more expensive and takes three times longer than for drug susceptible TB.¹² New advances in treatment strategies include the development of vaccines.
References
- WHO. TB country profile – Papua New Guinea. 2014. (https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCo untryProfile&ISO2=pg&outtype=pdf).
- Cross GB, Coles K, Nikpour M et al. TB incidence and characteristics in the remote gulf province of Papua New Guinea: a prospective study. BMC Infect Dis. 2014 Feb 20; 14:93.
- WHO. Guidelines for treatment of tuberculosis, fourth edition. 2010. (http://www.who.int/tb/publications/2010/9789241547833/en/)
- Van der Werf TS, Stienstra Y. Buruli ulcer (Mycobacterium ulcerans infection). UpToDate, 2016.
- Johnson PD, Stinear T, Small PL et al. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med. 2005 Apr; 2(4):e108.
- Etuaful S, Carbonnelle B, Grosset J et al. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005 Aug; 49(8):3182-6.
- Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007 Mar-Apr; 25(2):173-80.
- Van Zyl L, du Plessis J, Viljoen J. Cutaneous tuberculosis overview and current treatment regimens. Tuberculosis (Edinb). 2015 Dec; 95(6):629-38.
- Tseng HC, Lee CH, Cheng YW. Primary cutaneous tuberculosis in an elderly man. QJM. 2014 Dec; 107(12):1043-4.
- Ramesh V, Murlidhar S, Kumar J, Srivastava L. Isolation of drug-resistant tubercle bacilli in cutaneous tuberculosis. Pediatr Dermatol. 2001 Sep-Oct; 18(5):393-5.
- Ramesh V, Sen MK, Sethuraman G, D’Souza P. Cutaneous tuberculosis due to multidrug-resistant tubercle bacilli and difficulties in clinical diagnosis. Indian J Dermatol Venereol Leprol 2015; 81:380-4.
- Manjelievskaia J, Erck D, Piracha S, Schrager L. Drug-resistant TB: deadly, costly and in need of a vaccine. Trans R Soc Trop Med Hyg. 2016 Mar; 110(3):186-91.
- Sloan DJ, Lewis JM. Management of multidrug-resistant TB: novel treatments and their expansion to low resource settings. Trans R Soc Trop Med Hyg. 2016 Mar; 110(3):163-72.
- Rahman A, Sahrin M, Afrin S et al. Comparison of Xpert MTB/RIF Assay and GenoType MTBDRplus DNA Probes for Detection of Mutations Associated with Rifampicin Resistance in Mycobacterium tuberculosis. PLoS One. 2016 Apr 7; 11(4):20152694.
- Sharma SK, Kohli M, Yadav RN et al. Evaluating the Diagnostic Accuracy of Xpert MTB/RIF Assay in Pulmonary Tuberculosis. PLoS One. 2015 Oct 23; 10(10):20141011.



















































