Main content
Case
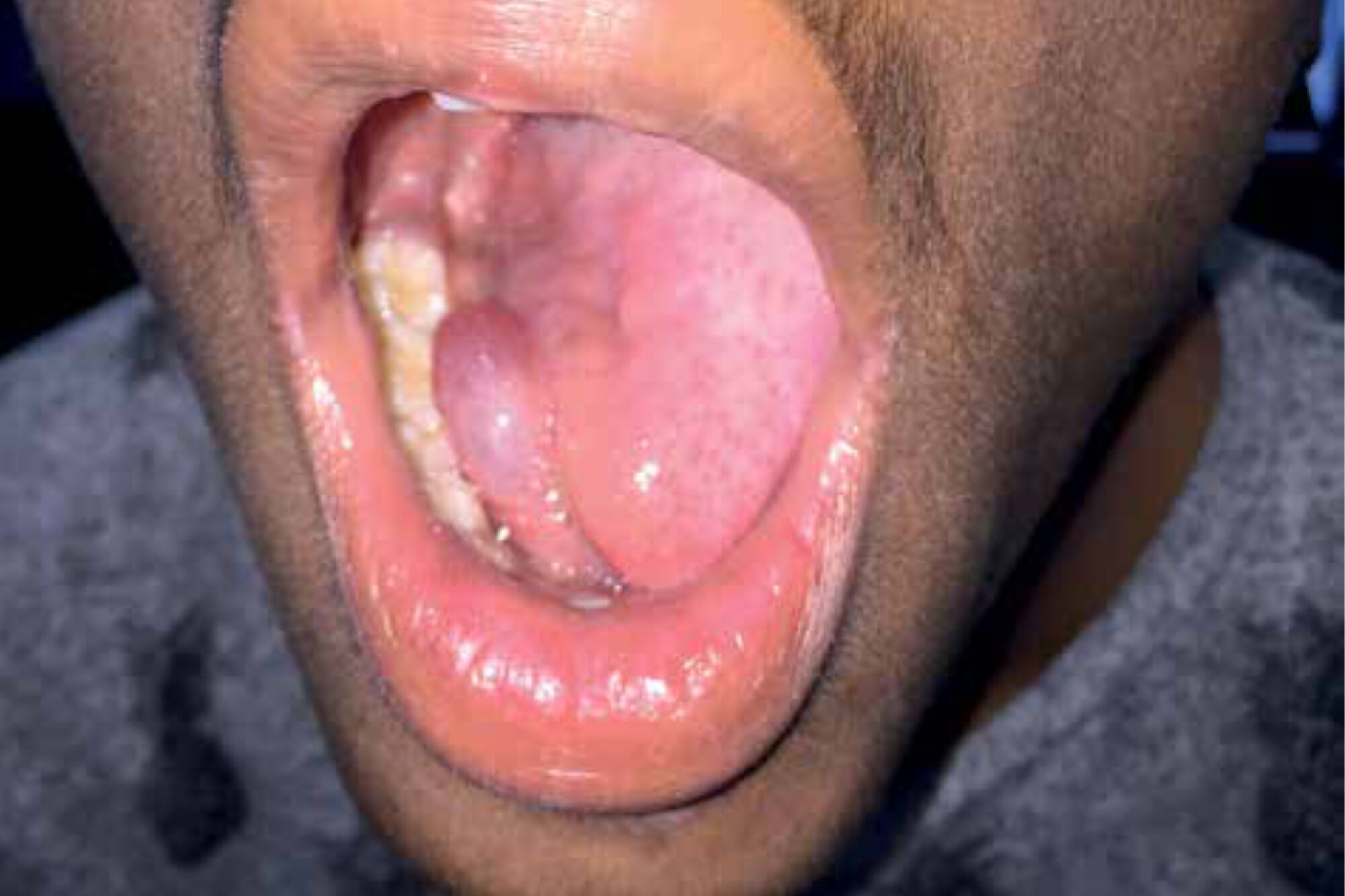
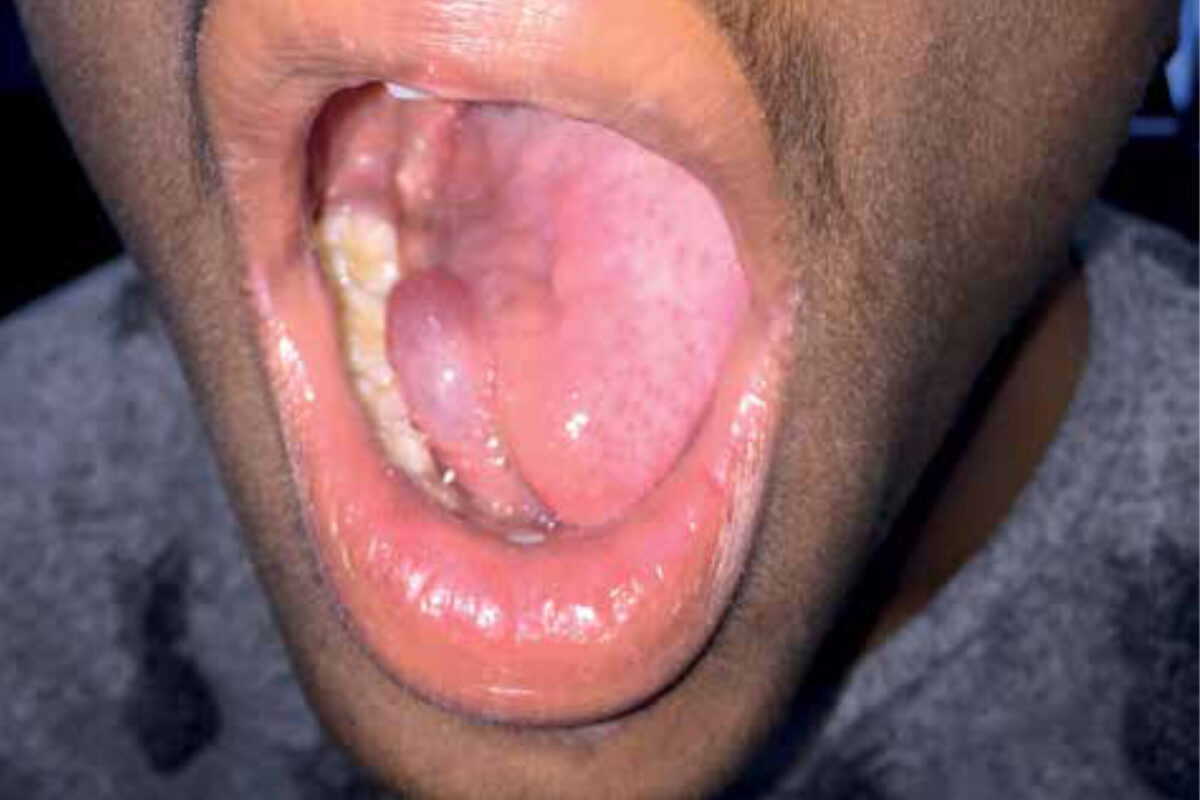
Late afternoon, at the end of outpatient clinic consultation hours in the Holy Mariam de Lourdes Hospital in Attat, Ethiopia, a 42-year-old male presented to our Ear-Nose-Throat (ENT) team with a large swelling of his tongue and floor of the mouth. We observed a sick, feverish patient with some shortness of breath who complained of difficulty swallowing and inability to move his tongue. ENT-examination revealed a large intra-oral swelling of the tongue, induration of the floor of the mouth and extensive dental caries (Figure 1).
Because of the limited resource setting, there was no access to radiodiagnostic equipment to help estimate the extent of the swelling, nor was mechanical ventilation available in case the airway would become further compromised and needed to be secured. Transferring the patient to the nearest hospital with these resources would mean an eight-hour drive to the capital city, which was not an option because of the risk of acute airway obstruction. Therefore, the ENT-team decided to locally drain the submental space in an attempt to reach adequate source control.
Before incision, fibreoptic laryngoscopy was performed which showed oedema of both the epiglottis tip and tongue base. Both vocal cords could be visual-ized, and the airway was sufficiently patent. The patient received ten milli-grams of dexamethasone intravenously, while a surgical set for a cricothyrotomy was constituted from several available surgical instruments. After a median incision in the floor of the mouth a large collection of pus was evacu-ated. There was no sign of immediate respiratory distress after surgery and the patient was admitted to the male ward. Six hours after local drainage of the submental space, the patient had died, probably due to a cardiac arrest. Resuscitation was of no help. For the ENT-team, this complication had an enormous impact and raised questions as to how this case could have been managed better. Should the airway have been secured first, maybe even with a tracheotomy, despite laryngos-copy before incision and in the absence of ventilation? This case report provides the clinical characteristics of Ludwig’s angina, including key factors in diagnosis and treatment, and presents a differential diagnosis of (sub)acute lesions in the floor of the mouth – all in the perspective of low-resource settings.
Background
Ludwig’s angina (LA), first described by Karl Friedrich Wilhelm von Ludwig in 1836, is caused by a rapidly spreading infection in the floor of the mouth. In up to 90% of all cases, this infection is caused by odontogenic foci, especially peri-apical abscesses of the second and third mandibular molars. Non-odontogenic causes include peritonsil-lar abscess, mandibular fracture and sialoadenitis.[1,2] In children, upper respiratory tract infections account for the majority of cases of LA.[3] Factors predisposing an individual to a higher risk of developing LA are poor dental hygiene, malnutrition and conditions that influence the immune system such as diabetes mellitus, acquired immune deficiency syndrome (AIDS) and the use of immunosuppressants.[4,5] In low- and middle-income countries (LMICs), mor-tality is still high, with rates up to 50% if patients do not receive timely and appro-priate treatment.[2] Several complications can occur such as necrotizing fasciitis of the neck, mediastinitis, pericarditis, rupture of the carotid artery or jugular vein thrombosis, pneumonia or pleural empyema. Post-operative complications occur in about a quarter of all patients.[5]
Anatomy and pathophysiology
The submandibular space is bound superiorly by the mucosal layer of the floor of the mouth and inferiorly by the superficial layer of the deep cervical fas-cia. The mylohyoid muscle divides the submandibular space in two spaces: (i) the sublingual space located supe-rior to the muscle and (ii) the submy-lohyoid space inferior to the muscle and superior to the hyoid bone.[6] Both spaces have a close relation with the roots of the mandibular molars and they communicate at the posterior margin of the mylohyoid muscle. Periapical infections of the roots of the second and third molars drain into the submylohyoid space; the first and premolars root apices drain into the sublingual space.[3,7] Infection in the submylohyoid space can spread in a superior and posterior direction causing tongue enlargement to two or three times its original size with risk of hypopharyngeal obstruction.[2,6] The infection can then spread along the fascial planes towards the parapha-ryngeal and retropharyngeal space, through which it can reach the medias-tinum and cause e.g. mediastinitis.[3,6]
Since LA mostly originates from an odontogenic source, the infec-tion is mainly caused by polymicro-bial, oral flora which consist of both aerobic and anaerobic species.[3] The most common pathogens isolated from LA patients are Streptococcus viridans (40%), Staphylococcus aureus (27%) and Staphylococcus epidermidis (23%).[2] Klebsiella spe-cies are more common in diabetic or immunocompromised patients.[8] Together with improved oral hygiene, the global availability of antibiotics has led to a significant reduction in mortality from LA, which highlights antibiotic use as a cornerstone in the treatment of Ludwig’s angina.[4,8]
Clinical presentation
Patients typically present with systemic symptoms such as fever, malaise and dehydration resulting from reduced oral intake.[3] Frequent complaints include pain in the floor of the mouth and throat, dysphagia, dysphonia, trismus, and drooling.[8] Physical examination may reveal a firmly indurated floor of the mouth, bilateral swelling of the neck and an oedematous tongue, which can be displaced towards the palate, and hypopharynx. More advanced symptoms are inspiratory stridor, cyanosis and dys-pnoea, indicating airway obstruction.[2,3]
Diagnostic testing
The diagnosis of Ludwig’s angina is largely made upon clinical presen-tation.[9] Laboratory testing has no additional value, but blood samples should be taken before starting anti-biotic treatment. A CT-scan with intravenously administered contrast may help in estimating the extent of infection[2] but is usually not avail-able in limited resource settings.
Ultrasound is widely available and, in contrast to computed tomography, can be used in an upright position, which is more favourable in case of respira-tory distress.[2] Ultrasonography can be used to screen for hypo-echoic lesions that may suggest abscess formation.[10] Furthermore, it can localize swelling to deeper tissue planes, thereby detecting early oedema and infec-tion, assessing airway involvement, and estimating the diameter of the subglottic upper airway.[10,11]
Treatment
Assessing and securing the airway is the first and most important step in the treatment of LA. Especially since airway oedema can rapidly develop with spreading infection, the clini-cian should be prepared for immedi-ate airway intervention by means of endotracheal intubation or tracheos-tomy.[2,9] An ENT-surgeon should be consulted in an early phase to determine whether surgical decompression is indicated for primary source control.[3,9]
As mentioned before, administration of antibiotics is key in treating LA. Specific regimens vary widely in the literature, but it is advised to use broad-spectrum antibiotics covering both aerobic and anaerobic pathogens, such as penicillin combined with clindamy-cin or metronidazole.[3,5] The role of corticosteroids in the treatment of LA is still debated, but they may reduce airway oedema[9] and enhance antibi-otic penetration[2], thereby decreasing the need for an artificial airway as well as the length of hospital stay.[12]
Differential considerations
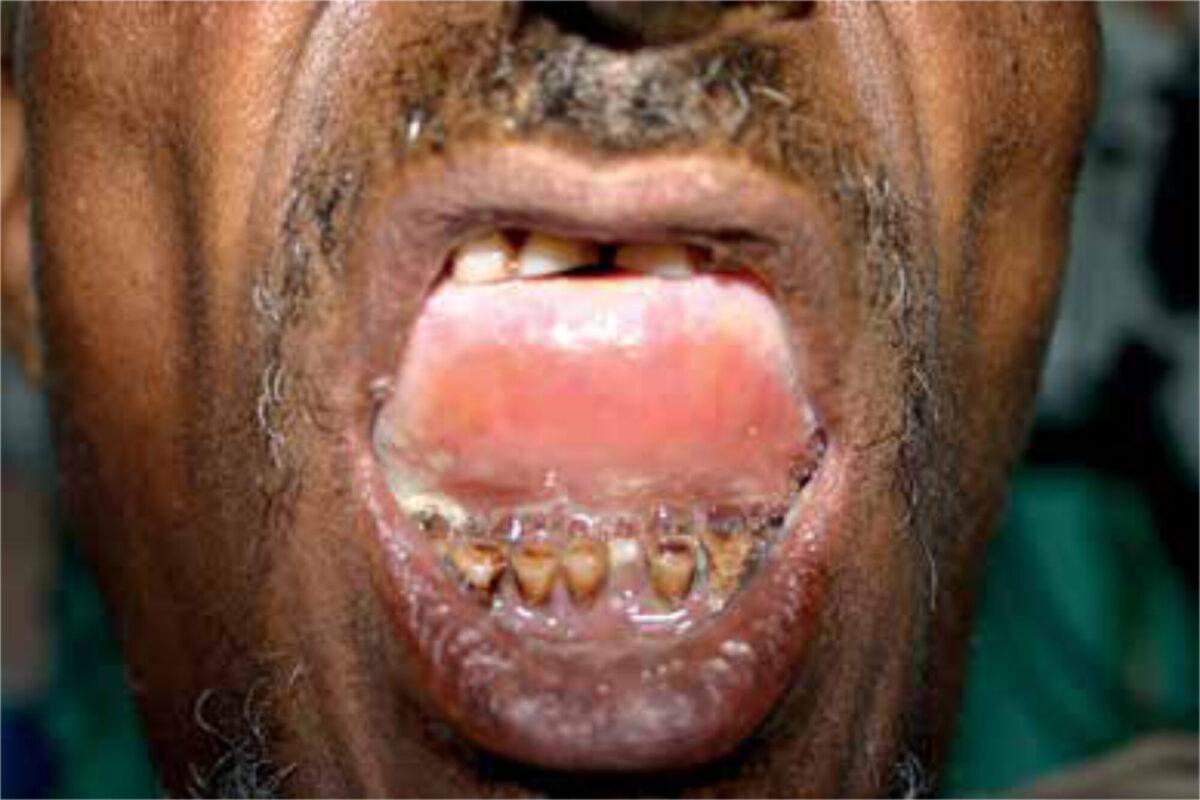
Table 1 presents an overview of the most common lesions in the floor of the mouth. Cystic lesions are usually slow-growing and benign and arise from salivary glands, mostly the sublingual gland. Most cystic lesions are ranu-las, which are mucous retention cysts with a bluish appearance (Figure 2). Plunging, or diving, ranulas present as a large submandibular swelling due to a defect in the mylohyoid muscle through which the cyst can enter the submandibular space. Dermoid cysts usually contain skin appendages and, as opposed to ranulas, do not have a bluish appearance. Other cystic lesions pres-ent lower down in the neck, generally below the level of the hyoid bone, and are related to branchial arch anoma-lies or thyroglossal duct remnants.
Inflammatory lesions, of which the above discussed Ludwig’s angina is one, constitute another group of lesions within the submandibular or sublingual space. The submandibular gland drains inferior to the tongue via the subman-dibular duct (Wharton duct). Calculi formed within the gland may obstruct the duct, thereby leading to enlargement and inflammation of the submandibular gland, a condition referred to as sialad-enitis. In 85% off the cases of subman-dibular gland enlargement, calculi are present in the duct. When calculi are not found, especially in older patients, a malignancy should be considered.
Generally, neoplastic lesions in the floor of the mouth are benign, with pleomorphic adenomas, especially of the submandibular gland, being the most frequently encountered. However, in a smaller gland such as the sub-lingual gland, enlargement is more likely resulting from a malignancy. Squamous cell carcinomas (SSCA) constitute up to 90% of all malignant lesions. It usually affects men over the age of 45 years and is related to tobacco and alcohol use. These lesions are in general ulcerated and infiltrative.
| CYSTIC LESIONS Ranulas (simple or plunging) Dermoid cyst Thyroglossal duct Branchial cleft cysts INFLAMMATORY NEOPLASTIC LESIONS VASCULAR LESIONS |
Conclusion
Ludwig’s angina is an acute and potentially life-threatening infection of the floor of the mouth that can rapidly spread, thereby being able to compro-mise a patient’s airway. Diagnosis is usually through history taking and clinical examination. Early recogni-tion of Ludwig’s angina is crucial, and airway protection is vital in the treat-ment of LA. In LMICs, where otorhino-laryngology and anaesthesia are often not readily available for consultation and intensive care units are not widely present, quick referral to a hospital with abovementioned services should be considered. In the presented case of Ludwig’s angina, the clinical course was fatal both as a result of the limited resource setting as well as the high rate of severe postoperative complica-tions known to be related to LA.
Acknowledgements
Prof.dr G. Isaacson for providing figure 2
References
- McDonnough JA, Ladzekpo DA, Yi I, Bond WR, et al. Epidemiology and resource utilization of ludwig’s angina ED visits in the United States 2006–2014. Laryngoscope. 2019;129(9):2041-2044. doi:10.1002/lary.27734
- Bridwell R, Gottlieb M, Koyfman A, Long B. Diagnosis and management of Ludwig’s angina: An evidence-based review. Am J Emerg Med. 2021;41:1-5. doi:10.1016/j.ajem.2020.12.030
- Lin HW, O’Neill A, Cunningham MJ. Ludwig’s angina in the pediatric population. Clin Pediatr (Phila). 2009;48(6):583-587. doi:10.1177/0009922809333095
- Dowdy RAE, Emam HA, Cornelius BW. Ludwig’s angina: Anesthetic management. Anesth Prog. 2019;66(2):103-110. doi:10.2344/anpr-66-01-13
- Botha A, Jacobs F, Postma C. Retrospective analysis of etiology and comorbid diseases associated with Ludwig’s Angina. Ann Maxillofac Surg. 2015;5(2):168. doi:10.4103/2231-0746.175758
- Pandey M, Kaur M, Sanwal M, et al. Ludwig’s angina in children anesthesiologist’s nightmare: Case series and review of literature. J Anaesthesiol Clin Pharmacol. 2017;33(3):406-409. doi:10.4103/0970-9185.214318
- Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis. 2009;13(3):327-333. doi:10.1016/j.ijid.2008.07.007
- Marcus BJ, Kaplan J, Collins KA. A case of Ludwig angina: A case report and review of the literature. Am J Forensic Med Pathol. 2008;29(3):255-259. doi:10.1097/PAF.0b013e31817efb24
- Costain N, Marrie TJ. Ludwig’s angina. Am J Med. 2011;124(2):115-117. doi:10.1016/j.amjmed.2010.08.004
- Gaspari RJ. Bedside ultrasound of the soft tissue of the face: A case of early Ludwig’s angina. J Emerg Med. 2006;31(3):287-291. doi:10.1016/j.jemermed.2005.11.044
- Lakhal K, Delplace X, Cottier JP, et al. The feasibility of ultrasound to assess subglottic diameter. Anesth Analg. 2007;104(3):611-614. doi:10.1213/01.ane.0000260136.53694.fe
- Busch RF, Shah D. Ludwig’s angina: Improved treatment. Otolaryngol Head Neck Surg. 1997;117(6): S172-S175. doi:10.1016/S0194-5998(97)70093-7