Main content
Back in 1999, a team of international experts was brought together as the DND Working Group of Médecins Sans Frontières (MSF) to study the crisis in research and development (R&D) for neglected diseases. Of the total of 1,393 approved products developed between 1975 and 1999, a mere 1.1% were for neglected diseases despite their accounting for a global disease burden of 12%, a so-called “fatal imbalance”. Although progress has been made, over the following decade still only 4% of all approved new products (reformulations, combinations and vaccines) and 1% of all approved new chemical entities were indicated for neglected diseases; with little change in their global health burden (estimated at 11%), the fatal imbalance persists.
Focus on kinetoplastida
Product development partnerships were initiated 15 years ago and include the Drugs for Neglected Diseases initiative (DNDi), created in 2003 as a collaborative, patients’ needs-driven, non-profit drug R&D organization. DNDi established an innovative model to develop products that may not otherwise have been developed, fill gaps in translational research, and build and strengthen R&D capacity in countries where neglected tropical diseases (NTDs) are endemic. The organization has had a strong focus on developing treatments for kinetoplastid diseases – sleeping sickness, leishmaniasis and Chagas disease – with further projects addressing filarial worm infections (causing onchocerciasis and lymphatic filariasis), the development of an affordable, taste-masked HIV treatment adapted for the very young, and two antimalarials.
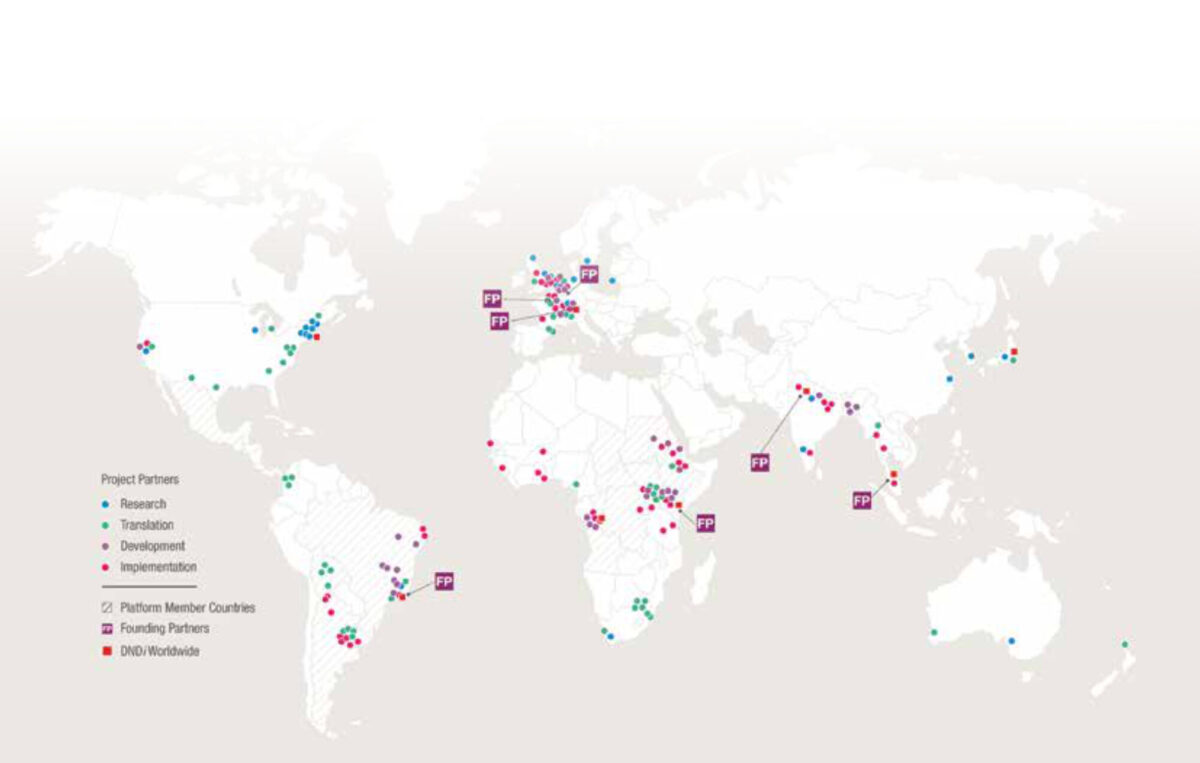
The innovative R&D model works through partnerships forged with the international research community in the public and private sector, who are involved at all stages of the development process. In early drug discovery stage, compounds originating from academia and pharmaceutical or biotechnology companies are screened for parasitological activity at standard “reference centres” before undergoing preclinical development, often by contract research organizations. Clinical trials are carried out in partnership with international and local disease experts. Clinical research platforms build and strengthen local capacity in endemic countries by bringing non-governmental organizations, academia, regulatory authorities and national control programmes together. Such platforms now exist in sub-Saharan and eastern Africa for sleeping sickness and leishmaniasis respectively, and in Latin America for Chagas disease. Platform members are involved from the outset, contributing to the development of target product profiles in line with patients’ needs. Local personnel are trained to conduct patient trials in line with international standards, and improvements to infrastructure are usually necessary. For example, treatment and diagnostic facilities have been built or renovated and equipped for sleeping sickness and leishmaniasis patients. The platforms are also able to facilitate registration and accelerate implementation of new treatments, working with national disease control programmes to ensure uptake of treatments in-country and advocating for policy change.
Six successes, with more to follow
By adapting and improving on existing treatments, six treatments have been successfully developed by DNDi and partners since 2003: ASAQ and ASMQ for malaria (fixed dose combinations of Artesunate + Amodiaquine and Artesunate + Mefloquine, respectively), NECT for sleeping sickness (NifurtimoxEflornithine Combination Therapy), SSG&PM for visceral leishmaniasis (VL), a set of treatments for VL in Asia, and a paediatric dosage form of benznidazole for Chagas disease. In addition, the results of longer term research are now also bearing fruit, with a number of entirely new chemical entities from early drug discovery efforts now populating the research portfolio. Two such compounds currently undergoing clinical development are fexinidazole and SCYX-7158, through collaboration with Sanofi and Anacor respectively, for sleeping sickness.
The development of the two fixed dose combinations (FDC) for the treatment of malaria were the first projects DNDi undertook. They were designed to be affordable, patient-adapted antimalarial treatments, able to resist the demands of a tropical environment, and to be easy to administer, particularly to children. An innovative, stable formulation of the ASAQ FDC was developed with early development partners before Sanofi, the industrial development partner, signed an agreement to develop the combination as a non-patented product at a target price of less than one US dollar per treatment in 2005. The product has since been recognized by the WHO prequalification programme, an assurance of high quality which is used as a guide by international procurement agencies that bulk-purchase medicines for distribution in resource-limited countries. An innovative Risk Management Plan to monitor safety in the field was developed with Sanofi and submitted as the first of its kind to the World Health Organization, comprising a series of trials to gather data on the efficacy and safety of ASAQ FDC in patients in a variety of geographical settings. Studies have been undertaken with MSF, Epicentre, Medicines for Malaria Venture (MMV) and others. The treatment is currently registered for use in 30 African countries, as well as in India, Ecuador and Colombia, and it is the second most widely used treatment in Africa, with over 400 million treatments distributed to date. Both antimalarial projects, ASAQ and ASMQ, were formally handed over in May 2015 to the Access and Product Management Team of MMV, in order to continue efforts to help maximize patient access.
Risk sharing
Having a strong pharmaceutical partner is key to success. Projects are managed by joint development committees, with DNDi in charge of the clinical development through its regional clinical platforms, and the industrial partner responsible for manufacturing, registration and distribution. Such partnerships are mutually beneficial: DNDi gains access to expertise in drug development, manufacturing and registration, access to compound libraries for screening, and in-kind expertise along the development chain, whilst pharmaceutical companies are able to fulfil their social corporate responsibility commitments. More than this however, with this new business model, companies are now working in non-traditional areas enabling them to expand their scientific know-how – which may be applied in other more commercially viable areas of research – and to establish links with new partners. The risks associated with the development of a new product are shared and access to new markets gained. This may, for example, result in the USA Food and Drug Administration’s Priority Review Voucher being granted in a scheme designed to incentivize the development of treatments and other products for NTDs or rare paediatric diseases, entitling the bearer to a priority review for any other product.
Diversified funding and multilateral partners
Sustainable funding of neglected disease research is vital. DNDi receives approximately half of its funding from the public institutional sector – such as governmental or international development aid agencies – and half from private donors or foundations. The Dutch Ministry of Foreign Affairs awarded two grants, between 2006 and 2014, and negotiations for a five-year grant are currently being finalised. DNDi tries to maintain its financial independence by pooling resources from diverse funders, limiting donations to a maximum of 25% from any one donor, and minimizing earmarked donations. Approximately €380 million has been raised to date.
As a virtual research organization, DNDi has approximately 150 staff members, half of whom are based in endemic countries, and a further 600 people working on DNDi projects. A global network of over 130 partnerships has been developed, mostly in endemic countries, involving academia, pharmaceutical and biotechnology companies, international and non-governmental organizations, health ministries, contract research organisations and others (see figure), spanning the entire drug development process and united around a shared vision. But partners are not service providers, and this multilateral approach to achieving a common goal takes time and requires mutual understanding and above all trust: face-to-face meetings are encouraged and lead to an increased awareness of a partner’s working culture and its restraints. It is also important to define operating rules and be clear about the decision making process from the outset in order to manage expectations. Clearly there is strength in working together – as the oft-quoted African proverb states, “If you want to go fast go alone. If you want to go far, go together”.
Future outlook
In October 2015, DNDi launched a new business plan, aiming to develop 16-18 treatments (including the existing six) by its 20th anniversary in 2023 [3]. By incorporating a more dynamic portfolio approach, the organization will be able to maintain its primary focus on the most neglected diseases, with the added possibility of extending the scope of diseases in response to future unmet or urgent needs.
Together with other public health experts, DNDi is advocating for a global biomedical R&D fund and mechanism, as current proposals to tackle R&D gaps are too fragmented and fail to adequately address affordability and patient access to treatments [4]. There is a need to delink the cost of R&D from product price and to integrate global health R&D monitoring, coordination and financing.
The recent award of the Nobel Prize for Physiology or Medicine, which was shared by William C. Campbell and Satoshi Omura for their discoveries concerning a novel therapy against infections caused by roundworm parasites and Youyou Tu for her discovery of a novel therapy against malaria, is a welcome recognition of valuable research conducted on neglected diseases. Meanwhile, new and improved treatments are still needed to alleviate the suffering of all neglected patients in the future.
For further information: http://www.dndi.org/about-us/overview-dndi.html
References
- MSF Access to Essential Medicines Campaign. Fatal Imbalance The Crisis in Research and Development for Drugs for Neglected Diseases.; 2001.
- Pedrique B, Strub-Wourgaft N, Some C, et al. The drug and vaccine landscape for neglected diseases (2000-11): a systematic assessment. Lancet Glob Heal. 2013;(13). doi:10.1016/S2214-109X(13)70078-0.
- Drugs for Neglected Diseases initiative. DNDi Business Plan 2015-2023. 2015. Available at: http://www.dndi.org/images/stories/pdf_publications/DNDi_Business_Plan_2015-2023.pdf.
- Balasegaram M, Bréchot C, Farrar J, et al. A Global Biomedical R & D Fund and Mechanism for Innovations of Public Health Importance. PLOS Med. 2015;12(5:e1001831):2-5. doi:10.1371.



















































