Main content
An effective HIV vaccine is still not available, even though almost 40 years have passed since the detection of the virus. Two major HIV types exist, HIV-1 and HIV-2, with the former being the more widespread and pathogenic one. The major challenge for an effective HIV-1 vaccine is its enormous viral diversity. Vaccine-induced immune response will have to be able to cope with this diversity in order to be broadly protective against circulating HIV-1 strains.
While most licensed antiviral vaccines consist of live-attenuated or inactivated forms of the pathogens, these approaches are deemed unsafe for HIV-1, as an attenuated form of the virus could revert back to a pathogenic form and inactivation would need to be extremely effective to ensure safety. Therefore, newer vaccine platforms such as protein subunit, DNA, or recombinant viral vector vaccines have been explored. Many vaccine approaches target components of the envelope spike that mediates viral entry into target cells. As the envelope spike is the only viral protein on the outside of the virus, it is the only protein relevant for the induction of neutralizing antibodies, i.e. antibodies that can inactivate the virus (Figure 1). Of over 200 HIV-1 vaccines that have been tested in phase I trials in humans, only a handful progressed to phase IIb and III clinical trials, and none have been a great success. [1] However, several breakthroughs in the fundamental understanding of HIV-1 biology and immunology have driven new directions in HIV-1 vaccine research with promising results in preclinical studies. In this article, we describe the bumpy research path towards the development of an HIV-1 vaccine.
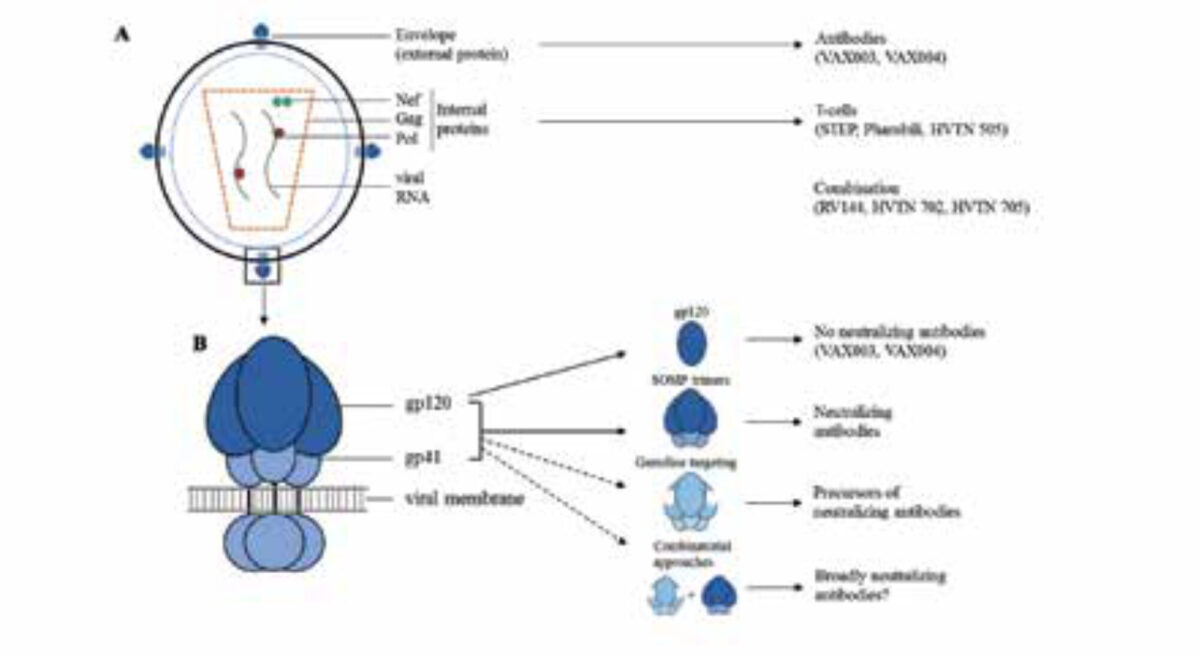
A. The HIV-1 particle. Indicated are the different viral proteins that have been used in different clinical trials.
B. The HIV-1 envelope glycoprotein trimer anchored in the cell membrane. Indicated are the gp120 and gp41 subunits, the monomeric gp120 vaccine candidate, the soluble cleaved native-like SOSIP.664 envelope trimer and the germline targeting trimer.
Early failures (1998 – early 2000s)
The initial focus of HIV-1 vaccine research was on inducing humoral immunity via antibodies. Two phase III trials, VAX004 and VAX003 that started in 1998 and 1999, in the US and Thailand respectively, were designed to induce neutralizing antibody responses. Both studies tested a recombinant gp120 protein, one of the subunits of the HIV-1 envelope spike (Figure 1). Both vaccines failed to induce neutralizing antibodies and showed no reduction in the percentage of individuals that acquired HIV-1 over time compared to placebo recipients. [1]
After the failure of these antibody-focused vaccines, the attention shifted to cellular immunity via T cells. Two trials, the STEP and Phambili trials, tested a vaccine consisting of a weakened recombinant form of a common cold virus, adenovirus type 5, expressing internal viral proteins to induce T-cell responses (Figure 1A). This vaccine also did not provide protection, and the trials were stopped prematurely because the vaccine was suspected to increase the risk of HIV-1 infection. [1] A third trial to test the T cell concept, HVTN505, used a vaccine that contained DNA plasmids encoding internal viral proteins and gp120 proteins. Similar to the previous studies, it did not show any efficacy against HIV-1 acquisition. [1]
A moderate success and lessons learned (2009 – now)
While the above vaccines aimed to induce either humoral or cellular immunity, simply combining such vaccines led to the first success, albeit a very modest one. The famous RV144 trail, conducted in Thailand, remains the only HIV-1 vaccine trial to demonstrate efficacy against HIV-1 acquisition. The trial evaluated a prime-boost vaccine that was composed of a recombinant non-replicating canary pox vector encoding internal viral proteins, followed by a gp120 protein boost (Figure 1). The study showed an efficacy of 31% protection against HIV-1 acquisition and raised hopes that generating a successful HIV-1 vaccine might be feasible. [2] To improve the quality, quantity and durability of the antibodies associated with the protection observed in the RV144 trial, a follow-up phase IIb trial, HVTN702, is currently being conducted in South Africa using geographically matched vaccine components. [3] A second phase IIb trial in sub-Saharan Africa, Imbokodo/HVTN705, also builds on the RV144 results and uses a recombinant adenovirus type 26 virus expressing immunologically optimized internal and envelope proteins, followed by booster immunizations with a geographically matched envelope protein. [4]
Aiming for broadly neutralizing antibodies
None of the above vaccines induce neutralizing antibodies against circulating virus strains – which is how most licensed viral vaccines work – let alone broadly neutralizing antibodies, i.e. antibodies that can neutralize diverse virus strains. This is a significant shortcoming, especially considering that passive immunization of monkeys with broadly neutralizing antibodies provides strong protection from virus acquisition. [5] Such broadly neutralizing antibodies do develop in approximately 20-30% of HIV-1 infected patients and some are able to neutralize more than 90% of the highly diverse circulating HIV-1 strains. This demonstrates that the human immune system can make such antibodies. Four complementary and exciting new routes aimed at inducing broadly neutralizing antibodies will be put to test in early phase clinical studies in 2018-2020.
Soluble envelope spike vaccines
The failure of previous HIV-1 vaccines is partly attributable to the fact that they only used a component of the envelope glycoprotein spike, monomeric gp120 or a misfolded version of it (Figure 1B). The gp120 subunit lacks a number of neutralizing antibody epitopes and also exposes immuno-dominant epitopes that serve as immune decoys. Over the last five years, significant progress has been made in generating better mimetics of the native envelope spike and the prototype mimetic is the BG505 SOSIP trimer. This stabilized envelope protein was the first to induce strong neutralizing antibody responses in animal vaccination studies, albeit only against the sequence-matched virus (6), and it will be evaluated in a phase I trial in 2019.
Lineage envelope vaccines
The broadly neutralizing antibodies in HIV-1 infected patients do not develop instantly in response to the incoming virus. Rather they usually emerge many years after the initial infection and are probably the product of continuous co-evolution of the immune-escaping viruses. [7] It is therefore unlikely that such antibodies will develop in response to any vaccine with a constant composition. The evolution of the broadly neutralizing antibody response has been extremely well documented in a patient named ‘CH505’ and revealed the underlying mechanisms between viral escape and antibody maturation. To recapitulate the co-evolutionary process that led to the development of broadly neutralizing antibodies in patient CH505, envelope vaccines based on the evolving viruses in that patient have been generated and a phase I clinical trial with sequential immunizations with these vaccines is now in progress. [7]
Activating b-cell precursors of broadly neutralizing antibodies
Sequential immunization may be necessary for antibodies to become broadly neutralizing antibodies, but an important step in such a strategy is for the first immunization to select for B-cells that have the intrinsic capacity to generate broadly neutralizing antibodies. [7] However, current envelope vaccines generally do not engage these desirable B-cells, so specifically engineered vaccines are needed. The most advanced vaccine candidate to achieve this is eOD-GT8. [7] A phase I trial has recently been started to test whether eOD-GT8 can select and activate the right precursor B cells in humans. A second vaccine designed to activate such B-cells is based on the BG505 SOSIP trimer and will enter human trials in 2019.[8]
Epitope-focused envelope vaccines
Envelope vaccines often display many potential distracting epitopes. Vaccines that are designed to focus the immune system on a particular epitope might resolve this problem and activate the desirable B-cells. An attractive candidate for epitope focusing is the envelope fusion peptide, as it is a relatively simple epitope. Vaccination of mice and monkeys using a fusion peptide vaccine, followed by a BG505 SOSIP trimer vaccine, resulted in the induction of neutralizing antibodies. [7] A human clinical trial following this concept is scheduled to start in 2019.
Concluding remarks
Ideally, an HIV-1 vaccine should induce high titres of broadly neutralizing antibodies to protect against virus infection, but no vaccine candidate is currently able to do so. Different approaches are being pursued in order to achieve this goal, with promising results in preclinical studies. A very promising approach involves targeted activation of desirable precursor B-cells of broadly neutralizing antibodies, a necessary first step. Furthermore, the SOSIP trimers are able to induce neutralizing antibodies in preclinical studies. None of the four strategies described above are mutually exclusive, and when used in combination they are likely to be employed more successfully (Figure 1B). After years of exploring the effectiveness of these separate approaches, there is hope that combining them will provide an effective protective HIV-1 vaccine.
References
- Mann JK, Ndung’u T. HIV-1 vaccine immuno-gen design strategies. Virol J 2015; 12: 3.
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209-20.
- Bekker L-G, Moodie Z, Grunenberg N, et al. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial. Lancet HIV 2018;5:e366-e378.
- Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet (London, England) 2018;392:232-243.
- Alter G, Barouch D. Immune Correlate-Guided HIV Vaccine Design. Cell Host Microbe 2018;24:25-33.
- Sanders RW, Moore JP. Native-like Env tri-mers as a platform for HIV-1 vaccine de-sign. Immunol Rev 2017;275:161-182.
- Kwong PD, Mascola JR. HIV-1 Vaccines Based on Antibody Identification, B Cell Ontogeny, and Epitope Structure. Immunity 2018;48:855-871.
- Medina-Ramírez M, Garces F, Escolano A, et al. Design and crystal structure of a native-like HIV-1 envelope tri-mer that engages multiple broadly neutralizing antibody precursors in vivo. J Exp Med 2017;214:2573-2590.

















































